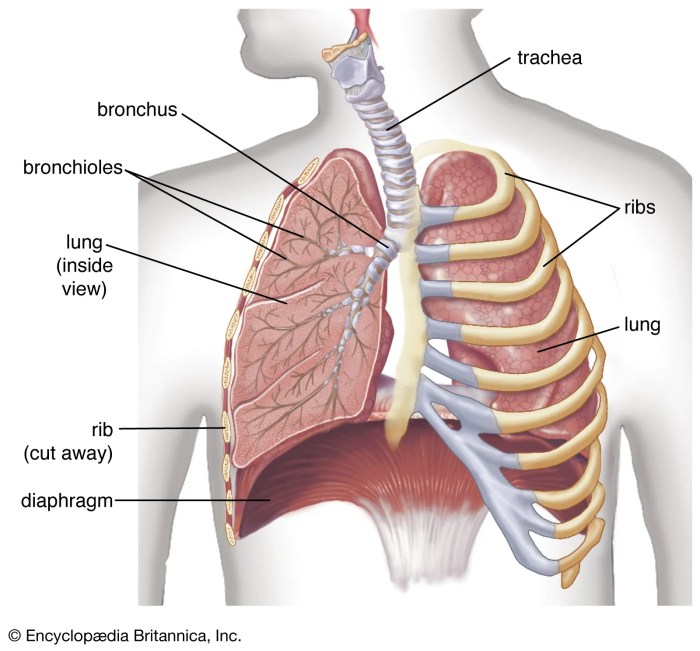
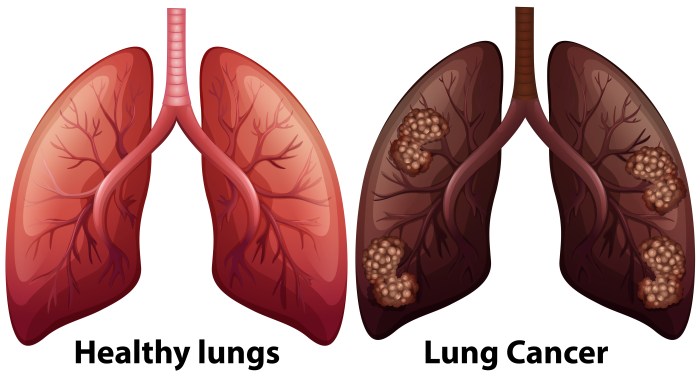
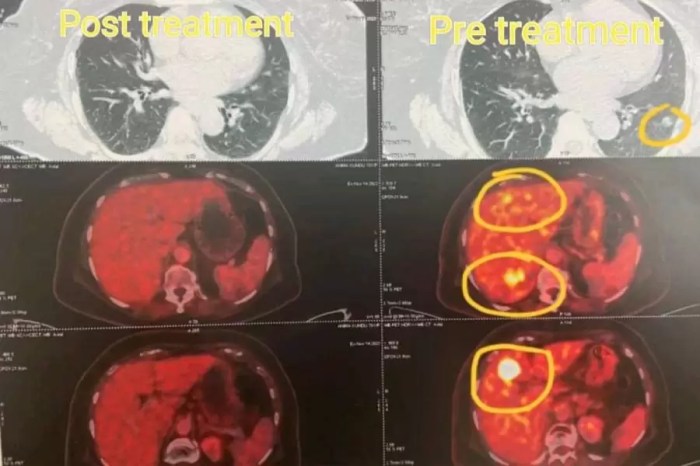
Maintenance therapy for lung cancer is a crucial aspect of treatment, aiming to prevent the cancer from returning or spreading. It’s a strategy that seeks to keep the cancer at bay after initial treatment, often by targeting specific mechanisms that drive the disease. This in-depth look explores the various types, mechanisms, patient considerations, protocols, side effects, and the ongoing research surrounding this vital approach to lung cancer care.
This exploration will delve into the science behind how these therapies work, who benefits most, and the challenges and future directions of maintenance therapy in the fight against lung cancer. We’ll also examine the patient experience and the support available to those undergoing this treatment.
Introduction to Maintenance Therapy for Lung Cancer
Maintenance therapy in lung cancer is a crucial component of treatment aimed at preventing the recurrence of the disease and improving overall survival. It’s designed to keep the cancer at bay after initial treatment, like surgery, chemotherapy, or radiation therapy, rather than solely focusing on eliminating existing tumors. This approach is particularly important for lung cancer, given its tendency to return even after apparent eradication.
Maintaining lung cancer treatment often involves a complex blend of therapies. While focusing on these crucial treatments, it’s also worth exploring natural methods for managing other health concerns. For instance, if you’re looking for ways to alleviate bunion discomfort, you might want to explore some of the natural remedies available, such as those outlined in this helpful guide on how to shrink bunions naturally.
Ultimately, a holistic approach to well-being can complement any cancer maintenance strategy.
It targets residual cancer cells that might not be visible after initial treatment, potentially preventing their growth and spread.The general goals of maintenance therapy in lung cancer encompass prolonged disease-free survival, minimizing the risk of relapse, and improving the quality of life for patients. Success hinges on identifying and targeting specific molecular pathways involved in cancer growth, thereby disrupting the cancer cells’ ability to proliferate.
This often involves the use of targeted therapies, immunotherapies, or even combinations of these, aiming to maintain a state of remission.
Different Types of Maintenance Therapies
Maintenance therapies for lung cancer are diverse, reflecting the complexity of the disease and the various molecular mechanisms driving its growth. They often involve medications that specifically target cancer cells, or boost the body’s own immune system to fight the cancer.
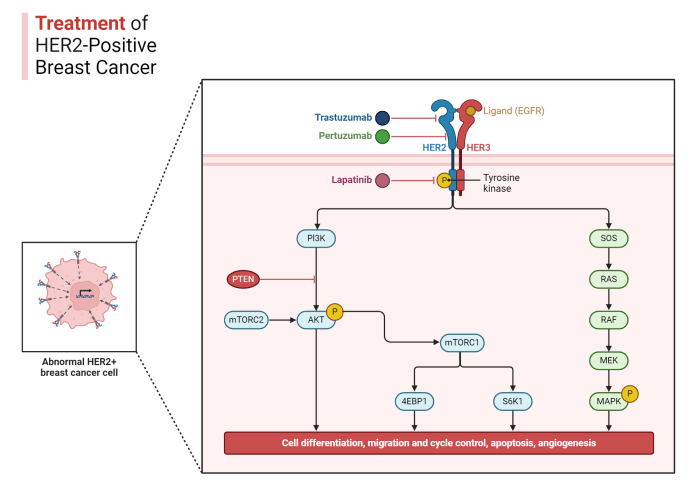
- Targeted Therapies: These medications act on specific proteins or pathways that are crucial for cancer cell growth. Examples include inhibitors of EGFR, ALK, or BRAF mutations, which are common in some lung cancers. These therapies can prevent the growth of residual cancer cells by disrupting their signaling pathways.
- Immunotherapies: These therapies harness the body’s own immune system to identify and destroy cancer cells. Immune checkpoint inhibitors, like pembrolizumab and nivolumab, are examples of immunotherapies frequently used in maintenance settings for lung cancer. They work by releasing the brakes on the immune system’s natural ability to recognize and attack cancer cells.
- Chemotherapy: In some cases, chemotherapy drugs, like pemetrexed or cisplatin, are used as maintenance therapy. These medications are still used to reduce the likelihood of cancer recurrence, but their use is often more targeted and tailored to specific patient characteristics and the type of lung cancer.
Efficacy and Side Effects Comparison
A comparison of maintenance therapies for lung cancer, while not universally conclusive, highlights the complex interplay between efficacy and potential side effects. No single therapy consistently outperforms others in every patient.
| Therapy Type | Efficacy (Example Outcomes) | Potential Side Effects |
|---|---|---|
| Targeted Therapies (e.g., EGFR inhibitors) | Can significantly reduce the risk of recurrence in specific lung cancer subtypes, especially those with EGFR mutations. Improved progression-free survival has been observed in some clinical trials. | Common side effects include skin rashes, diarrhea, and fatigue. More serious side effects like liver problems or lung inflammation are possible, but less frequent. |
| Immunotherapies (e.g., Immune checkpoint inhibitors) | Demonstrate promising results in improving overall survival in certain lung cancer types, especially non-small cell lung cancer. Studies have shown that maintenance immunotherapy can extend the time until cancer returns. | Common side effects include fatigue, nausea, and skin reactions. More severe side effects like immune-related adverse events (such as colitis or pneumonitis) can occur in a minority of patients. |
| Chemotherapy (e.g., pemetrexed) | Historically used as maintenance therapy, but its efficacy varies depending on the specific type of lung cancer and the patient’s overall health. | Chemotherapy maintenance often comes with a higher risk of side effects such as nausea, hair loss, and bone marrow suppression. The intensity of these side effects varies widely. |
Mechanisms of Action
Maintenance therapies for lung cancer aren’t just about slowing the disease; they aim to disrupt the underlying processes that fuel cancer growth and spread. Understanding the specific mechanisms these therapies employ is crucial for predicting patient responses and tailoring treatment strategies. Different drugs work through various pathways, impacting the cancer cells’ ability to multiply and metastasize. The selection of a maintenance therapy is highly individualized, considering factors like the specific type of lung cancer, the patient’s overall health, and the presence of biomarkers indicative of responsiveness.
Targeting Cellular Pathways
Maintenance therapies for lung cancer often target specific cellular pathways crucial for cancer cell survival and proliferation. These pathways, like the PI3K/Akt/mTOR pathway and the EGFR pathway, play a vital role in regulating cell growth and division. Disrupting these pathways can hinder the ability of cancer cells to reproduce and spread. For example, therapies targeting the EGFR pathway in non-small cell lung cancer (NSCLC) can significantly inhibit tumor growth.
Role of Biomarkers in Predicting Response
Biomarkers are essential tools in identifying patients who are likely to benefit from maintenance therapies. The presence or absence of specific biomarkers, such as specific mutations in the EGFR gene or the presence of PD-L1, can help predict a patient’s response to particular drugs. For instance, patients with EGFR mutations are more likely to respond to EGFR-targeted therapies.
Testing for these biomarkers before starting maintenance therapy allows for a more targeted and effective treatment approach. The availability of biomarker testing has revolutionized precision oncology, enabling clinicians to select the most appropriate maintenance therapy for each patient.
Impact on Cancer Growth and Spread
Maintenance therapies aim to reduce the growth and spread of lung cancer by interrupting the processes driving tumor development. This can involve inhibiting the growth of new blood vessels that feed the tumor (angiogenesis), reducing the ability of cancer cells to migrate and invade surrounding tissues (metastasis), or triggering programmed cell death (apoptosis) in cancer cells. For example, immunotherapy drugs can trigger the body’s immune system to recognize and destroy cancer cells, potentially preventing the resurgence of the disease.
Comparison of Mechanisms
Different maintenance therapies employ varying mechanisms to achieve their goals. Some, like targeted therapies, directly interfere with specific molecular pathways crucial for cancer cell growth. Others, such as immunotherapy agents, stimulate the body’s own immune system to attack and eliminate cancer cells. The choice of therapy depends on the specific genetic profile of the tumor and the patient’s overall health.
| Therapy Type | Mechanism of Action | Examples |
|---|---|---|
| Targeted Therapy | Inhibit specific molecular pathways in cancer cells | EGFR inhibitors, ALK inhibitors |
| Immunotherapy | Stimulate the immune system to recognize and destroy cancer cells | PD-1 inhibitors, CTLA-4 inhibitors |
Patient Selection and Considerations: Maintenance Therapy For Lung Cancer
Maintenance therapy for lung cancer, while offering a potential survival benefit, isn’t a one-size-fits-all approach. Careful patient selection is crucial to maximizing the effectiveness and minimizing the risks associated with this treatment. Factors such as the specific type of lung cancer, the patient’s overall health, and the response to initial treatment all play a significant role in determining eligibility.Understanding the specific criteria for eligibility, along with the role of comorbidities, is vital for ensuring the safety and efficacy of maintenance therapy.
Careful assessment and consideration of individual patient profiles are critical in achieving optimal outcomes.
Factors Influencing Patient Selection
Careful consideration of various factors is necessary to identify suitable candidates for maintenance therapy. These factors help ensure that the treatment is both beneficial and safe. Patients who have shown a favorable response to initial treatment, such as demonstrating tumor shrinkage or stable disease, are more likely to benefit from maintenance therapy. This positive response suggests the therapy may effectively control the cancer’s growth and spread.
Eligibility Criteria for Maintenance Therapy
Specific criteria dictate eligibility for maintenance therapy. These criteria often involve evaluating the patient’s performance status, the stage and type of lung cancer, and the results of prior treatment. The criteria aim to select patients most likely to derive a survival benefit from maintenance therapy. For example, patients with advanced-stage lung cancer, but who have shown a favorable response to prior chemotherapy, might be considered for maintenance therapy.
Patients who exhibit poor performance status or severe comorbidities might not be eligible due to potential treatment-related risks.
Role of Comorbidities in Patient Selection
Comorbidities, or existing health conditions, can significantly impact a patient’s ability to tolerate maintenance therapy. The presence of severe or uncontrolled comorbidities, such as significant cardiovascular disease, severe kidney disease, or severe liver disease, may increase the risk of adverse events and reduce the potential benefit of maintenance therapy. Careful assessment of the severity and stability of these conditions is essential.
For instance, a patient with stable heart failure might be eligible, whereas one with uncontrolled heart failure might not.
Potential Contraindications to Maintenance Therapy
| Potential Contraindication | Explanation |
|---|---|
| Uncontrolled comorbidities (e.g., severe heart failure, uncontrolled diabetes) | Patients with poorly controlled underlying conditions may experience increased toxicity and reduced tolerability to maintenance therapy. |
| Poor performance status (ECOG PS ≥ 2) | Patients with significant functional limitations may struggle to tolerate the treatment regimen and experience higher rates of adverse events. |
| Prior severe adverse events to prior chemotherapy | Patients who experienced severe side effects from prior chemotherapy regimens may be at higher risk for similar reactions during maintenance therapy. |
| Significant organ dysfunction (e.g., severe liver or kidney impairment) | Compromised organ function can hinder the body’s ability to process the medication, leading to potential toxicity. |
| Uncontrolled infections | Active infections can interfere with treatment efficacy and increase the risk of complications. |
Treatment Protocols and Procedures
Navigating the complexities of lung cancer maintenance therapy requires a deep understanding of treatment protocols. These meticulously designed plans dictate the specific drugs, dosages, and schedules for each patient, aiming to control the disease and improve quality of life. This section delves into the practical aspects of implementing these protocols, emphasizing the crucial role of adherence and the importance of personalized strategies.
Common Treatment Protocols
Different protocols exist, tailored to the specific type of lung cancer, the patient’s overall health, and the stage of the disease. A key consideration is the type of maintenance therapy employed. For example, some protocols might incorporate targeted therapies, while others might rely on chemotherapy or immunotherapy.
Administration Methods
The manner in which maintenance therapies are administered is just as critical as the drugs themselves. Oral medications are frequently used, providing convenience and allowing patients to manage their treatment at home. However, intravenous (IV) infusions, administered in a clinic or hospital setting, are sometimes necessary, especially for certain drugs. The method chosen depends heavily on the specific medication and its properties.
Dosage and Frequency
Dosage and frequency are meticulously determined by the oncologist based on the individual patient’s characteristics and the specific maintenance therapy regimen. Factors such as body weight, organ function, and the patient’s response to treatment are all considered. For instance, a higher dosage of a particular drug might be needed for a larger patient, while a lower dosage might be sufficient for a patient with compromised kidney function.
The frequency of administration, whether daily, weekly, or monthly, also depends on the characteristics of the medication.
Maintenance therapy for lung cancer is a crucial aspect of treatment, aiming to prevent recurrence. While it’s often focused on specific drugs and their side effects, understanding the nuances of different inflammatory bowel diseases, like the distinctions between Crohn’s disease (CD) and ulcerative colitis (UC), understand the differences between cd and uc , can be surprisingly relevant. This knowledge can help patients and doctors better manage potential side effects and overall treatment plans for lung cancer.
Importance of Adherence
Adherence to the prescribed treatment protocol is paramount for the success of maintenance therapy. Regular appointments, consistent medication intake, and timely follow-up are essential. Patients should actively participate in treatment decisions and communicate any concerns or side effects to their healthcare team. Missing doses or skipping appointments can significantly reduce the effectiveness of the treatment, potentially leading to disease progression.
For example, a patient who misses scheduled IV infusions may not receive the full dose of medication required for optimal therapeutic benefit. This underscores the importance of understanding and adhering to the prescribed schedule.
Example of a Treatment Protocol
| Drug | Dosage | Frequency | Route |
|---|---|---|---|
| Alectinib | 120 mg | Once daily | Oral |
| Bevacizumab | 15 mg/kg | Every two weeks | IV Infusion |
This table provides a simplified example. Actual protocols will be much more complex, considering many individual patient factors. Always consult with your healthcare provider for personalized guidance.
Side Effects and Management
Maintenance therapies for lung cancer, while crucial for long-term survival, can unfortunately come with a range of potential side effects. Understanding these side effects and developing strategies to manage them is vital for patient comfort and adherence to the treatment plan. This section delves into common side effects, management strategies, and the importance of consistent monitoring.
Common Side Effects
Maintenance therapies, like targeted therapies and immunotherapy, can impact various bodily systems. Recognizing these common side effects is essential for early intervention and proactive management. These side effects often vary in severity and duration depending on the specific therapy and individual patient factors.
Strategies for Managing Side Effects
Effective management of side effects is crucial for patient well-being and treatment adherence. A multi-faceted approach, incorporating medical interventions, lifestyle adjustments, and emotional support, often proves most beneficial. Early recognition and prompt intervention are key to minimizing the impact of these side effects.
Importance of Monitoring for Side Effects
Regular monitoring during maintenance therapy is critical. This allows healthcare professionals to detect any emerging or worsening side effects early on. Early detection facilitates timely adjustments to the treatment plan, potentially preventing severe complications. This proactive approach ensures the patient receives the best possible care and maintains an optimal quality of life.
Potential Side Effects and Management Strategies
| Potential Side Effect | Management Strategy |
|---|---|
| Fatigue | Regular rest periods, prioritizing sleep hygiene, potentially adjusting the maintenance therapy dose or schedule. Consider lifestyle modifications such as incorporating exercise (with doctor’s approval), and a balanced diet. |
| Nausea and Vomiting | Anti-emetic medications, dietary modifications (small, frequent meals, avoiding greasy or strong-smelling foods), and potentially adjusting the maintenance therapy schedule. |
| Diarrhea | Dietary adjustments (low-fiber diet initially), medications to control diarrhea, and ensuring adequate fluid intake. |
| Skin Rashes/Dermatitis | Moisturizing lotions, avoidance of harsh soaps or detergents, and close monitoring by the dermatologist, adjusting the maintenance therapy dose or switching to an alternative if necessary. |
| Constipation | Increasing fiber intake in the diet, increasing fluid intake, stool softeners, and regular bowel movements. |
| Hair Loss | No specific medication to reverse this. Emotional support, wigs, and turbans can help manage this distressing side effect. |
| Mouth Sores/Mucositis | Soft, bland foods, frequent mouth rinsing with saline or prescribed mouthwashes, and potential adjustments in the maintenance therapy. |
| Infections | Close monitoring for signs of infection, proactive use of preventative measures like vaccinations, and adjusting the maintenance therapy if necessary. |
| Neurological Effects (e.g., headaches, neuropathy) | Pain management strategies (analgesics), symptom monitoring, and adjustment of the maintenance therapy. |
Clinical Trials and Research
The quest for better lung cancer treatments, particularly maintenance therapies, heavily relies on ongoing clinical trials and research. These studies are crucial in evaluating the efficacy and safety of new approaches, leading to improvements in patient outcomes and ultimately, better care. Understanding the latest findings in this area is essential for informed decision-making in the field of oncology.
Ongoing Clinical Trials
Numerous clinical trials are currently underway, investigating various maintenance strategies for lung cancer patients. These trials often focus on different types of lung cancer, specific genetic markers, and diverse treatment regimens. A significant portion of these trials involve immunotherapy agents combined with targeted therapies or chemotherapy. The results of these studies are vital for tailoring treatment plans to individual patient needs.
Latest Research Findings
Recent research suggests that maintenance therapies, particularly those incorporating checkpoint inhibitors, can significantly prolong survival in specific lung cancer subtypes. For instance, studies have shown promising outcomes in patients with advanced non-small cell lung cancer (NSCLC) harboring specific genetic alterations. However, the findings are not uniform across all types of lung cancer, emphasizing the need for individualized treatment approaches.
Furthermore, the effectiveness of maintenance therapies varies depending on the specific treatment protocol, patient characteristics, and the type of cancer.
Role of Research in Improving Protocols
Research plays a critical role in refining maintenance therapy protocols. Clinical trials provide data on the optimal timing, duration, and combination of treatments. By analyzing the outcomes of these studies, researchers can identify the most effective strategies for maximizing treatment benefits while minimizing side effects. The meticulous analysis of patient responses allows for the development of personalized treatment plans, tailoring maintenance therapies to the individual needs of each patient.
Emerging Trends and Future Directions
Emerging trends in maintenance therapy research include exploring novel combinations of targeted therapies and immunotherapies. For example, researchers are investigating the potential of combining immune checkpoint inhibitors with other targeted therapies, such as EGFR inhibitors, to enhance anti-tumor activity. Future directions also involve investigating predictive biomarkers to identify patients who are most likely to benefit from maintenance therapies.
This personalized approach promises to significantly improve patient outcomes. One example of this future direction is the development of blood tests to determine which patients are likely to respond to certain maintenance therapies. This would allow for more targeted treatment selection, ultimately leading to more effective and less toxic treatments. Furthermore, research is focusing on the long-term effects of maintenance therapies, aiming to minimize late complications and improve quality of life for long-term survivors.
Patient Experiences and Support
Navigating a cancer diagnosis, especially lung cancer, is incredibly challenging. Maintenance therapy, while often crucial for extending survival and improving quality of life, presents unique emotional and practical hurdles for patients. Understanding the patient experience and providing robust support systems are vital components of effective cancer care.Living with the prospect of a chronic illness and the ongoing uncertainty of treatment can be emotionally taxing.
The need for consistent medical appointments, potential side effects, and the emotional toll of a life-altering diagnosis can create significant stress. This section explores the patient journey with maintenance therapy, highlighting the importance of emotional and psychological support and sharing real-world examples.
Patient Experience with Maintenance Therapy
The experience of undergoing maintenance therapy for lung cancer is diverse, reflecting individual patient needs and responses to treatment. Some patients experience minimal side effects and maintain a high quality of life throughout the treatment period, while others encounter significant challenges, including fatigue, nausea, or other symptoms. These experiences are often compounded by the emotional and psychological impact of a cancer diagnosis.
Maintenance therapy for lung cancer is a crucial part of treatment, aiming to prevent recurrence. While dealing with the side effects of this therapy, it’s important to consider over-the-counter cough remedies like comparing Delsym and Robitussin, which can help manage symptoms. Understanding the differences between these medications is key, as detailed in this helpful resource delsym vs robitussin how do they differ.
Ultimately, managing side effects alongside the maintenance therapy is essential for patient comfort and overall treatment success.
It’s crucial to recognize that the patient experience is not a one-size-fits-all scenario.
Importance of Emotional and Psychological Support
Providing comprehensive emotional and psychological support is paramount during maintenance therapy. This support includes addressing anxieties, fears, and concerns about the treatment’s effectiveness, potential side effects, and the future. Support groups, counseling, and access to trained professionals are critical in helping patients cope with the emotional burden of the disease. These resources are essential in empowering patients to manage their well-being throughout the treatment process.
Examples of Successful Patient Journeys
Several individuals have successfully incorporated maintenance therapy into their treatment plans, demonstrating resilience and adaptation. For instance, one patient, after initially struggling with fatigue, found significant improvement by incorporating regular exercise into their daily routine. A support group facilitated by a trained oncologist provided a safe space for emotional sharing and problem-solving, fostering a sense of community and reducing isolation.
These examples underscore the importance of individualized support and tailored strategies for managing the treatment experience.
Resources and Support Systems for Patients
Numerous resources and support systems can aid patients undergoing maintenance therapy. These include:
- Support Groups: Online and in-person support groups offer a platform for sharing experiences, providing encouragement, and fostering a sense of community among individuals facing similar challenges.
- Counseling Services: Access to trained counselors or therapists can help patients process their emotions, develop coping mechanisms, and navigate the emotional rollercoaster of a cancer diagnosis.
- Patient Advocacy Organizations: These organizations often provide valuable information, resources, and advocacy for patients’ rights and needs, advocating for their well-being and access to quality care.
- Financial Assistance Programs: Many organizations offer financial assistance to patients facing the financial burdens of cancer treatment, including maintenance therapy.
- Oncology Social Workers: These professionals offer vital support and guidance on navigating the complexities of cancer care, addressing emotional and practical needs.
These resources, when combined with personalized care plans, can create a robust support network that empowers patients to manage their health and well-being effectively.
Comparison with Other Therapies
Maintenance therapy for lung cancer offers a unique approach to treatment, contrasting with standard therapies like chemotherapy and radiation. It aims to prevent recurrence after initial treatment, rather than solely addressing the current tumor burden. Understanding its strengths and weaknesses in comparison with other approaches is crucial for informed patient decisions.Maintenance therapy differs significantly from upfront curative therapies in its timing and objective.
While upfront treatments directly target the primary tumor, maintenance therapy focuses on long-term prevention of recurrence. This shift in focus impacts the patient experience and the potential for side effects.
Maintenance Therapy vs. Chemotherapy
Maintenance therapy and chemotherapy both aim to control cancer, but their approaches and objectives differ. Chemotherapy directly targets rapidly dividing cells, including cancer cells, aiming to shrink tumors and destroy them. In contrast, maintenance therapy aims to prevent cancer cells from returning or growing, rather than killing them directly. This means maintenance therapy often uses lower doses of drugs or different mechanisms of action.Maintenance therapies often employ targeted agents that inhibit specific molecular pathways that drive cancer growth, thereby minimizing harm to healthy cells.
Chemotherapy, while effective in many cases, can have more significant side effects due to its broader action on all rapidly dividing cells. Patients might prefer maintenance therapy for its potential to reduce long-term side effects.
Maintenance Therapy vs. Radiation Therapy
Radiation therapy uses high-energy rays to destroy cancer cells. It is often used in combination with surgery or chemotherapy. Maintenance therapy, however, focuses on preventing recurrence after primary treatment, which may or may not include radiation. The difference in the focus means that radiation is often used as part of the initial treatment plan, while maintenance therapy may be added later to prevent relapse.The choice between radiation and maintenance therapy depends on the specific type and stage of lung cancer.
Patients who have already undergone radiation might find maintenance therapy beneficial to prevent the recurrence of the cancer. It’s crucial to understand that radiation therapy often targets the area of the tumor directly, while maintenance therapy often targets specific molecular pathways.
Potential for Combining Therapies
Maintenance therapy can be combined with other therapies to enhance its effectiveness. For example, combining targeted agents used in maintenance therapy with checkpoint inhibitors can enhance the overall response rate. Combining maintenance therapy with other treatment strategies can maximize the effectiveness of cancer control and potentially reduce the risk of relapse.One example of combining maintenance therapy with other strategies involves using immunotherapy in conjunction with targeted agents.
This combination can lead to a stronger anti-tumor effect. However, the specific combination and its suitability for each patient require careful consideration by the medical team. The decision to combine maintenance therapy with other treatments will depend on individual patient factors and the type of lung cancer.
Situations Favoring Maintenance Therapy
Maintenance therapy might be a preferred option in situations where the risk of recurrence is high, such as in early-stage lung cancers or after successful treatment. It can also be beneficial for patients who are at high risk of recurrence but are not able to tolerate the side effects of more aggressive therapies.For example, patients with certain genetic mutations might benefit from maintenance therapy to prevent tumor regrowth, particularly if they have a strong history of recurrence in their family.
In some cases, maintenance therapy could offer a more tolerable alternative to repeated chemotherapy cycles.
Future Directions and Challenges
Maintenance therapy for lung cancer is rapidly evolving, driven by ongoing research and a growing understanding of the disease’s complexities. This dynamic field presents exciting opportunities for improving outcomes, but also significant challenges in implementation and patient selection. The future hinges on refining current strategies and developing novel approaches that address the limitations of existing therapies.
Potential Advancements in Maintenance Therapies
The quest for more effective and personalized maintenance therapies is fueled by ongoing research. Scientists are exploring new targeted therapies, focusing on specific molecular pathways implicated in lung cancer development and progression. Immunotherapy, with its potential to enhance the body’s own defenses against cancer cells, is another area of intense investigation. These innovative approaches aim to improve response rates, prolong survival, and minimize the side effects associated with traditional chemotherapy.
Future Directions in Targeted Therapies
The identification of specific genetic alterations in lung cancer cells is driving the development of targeted therapies. For instance, therapies targeting EGFR mutations, a common driver mutation in non-small cell lung cancer, have shown promise in extending survival. Further research is focused on developing therapies that address resistance mechanisms, improving the efficacy of these agents, and exploring combinations with other targeted therapies.
Challenges in Implementing Maintenance Therapy, Maintenance therapy for lung cancer
Implementing maintenance therapy effectively faces challenges related to patient selection and treatment adherence. Identifying patients most likely to benefit from maintenance therapy requires robust biomarkers and predictive models. Furthermore, the complex side effect profiles of some therapies and the potential for drug interactions necessitate careful patient monitoring and management. The cost of these advanced therapies also poses a significant barrier for some patients and healthcare systems.
The Need for Ongoing Research and Development
The field of maintenance therapy for lung cancer demands ongoing research to address current limitations and optimize treatment strategies. Clinical trials are essential for evaluating new therapies, assessing their efficacy, and refining their use in specific patient populations. This continuous investigation is vital to personalize treatment plans, improve patient outcomes, and ensure the long-term sustainability of these therapies.
Future Outlook for Maintenance Therapy
The future outlook for maintenance therapy in lung cancer is promising, yet complex. While existing therapies have shown positive results in extending survival and improving quality of life, significant challenges remain. The development of more precise biomarkers, the exploration of novel therapies, and the integration of personalized medicine approaches will be critical in advancing the field. Continued investment in research and clinical trials is essential to ensure that maintenance therapy plays a pivotal role in improving the lives of lung cancer patients.
Last Recap
In conclusion, maintenance therapy for lung cancer represents a promising avenue in improving outcomes for patients. While challenges remain, ongoing research and development, coupled with a personalized approach to patient selection, are paving the way for more effective and less toxic therapies. Understanding the various aspects of maintenance therapy is crucial for patients, caregivers, and healthcare professionals alike to make informed decisions about this important treatment option.