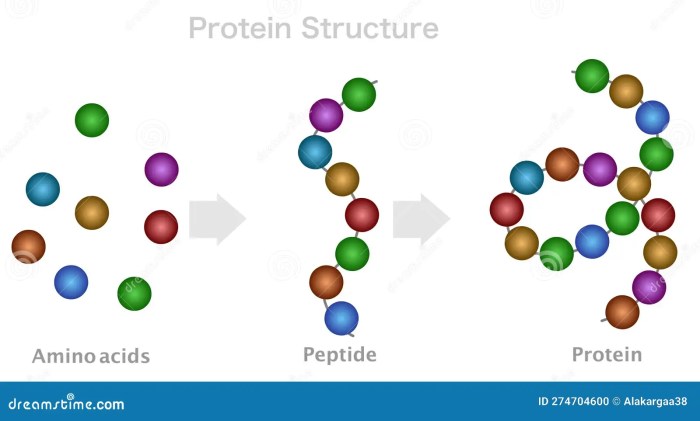
What is a peptide? This exploration delves into the fascinating world of peptides, those short chains of amino acids that play crucial roles in various biological processes. From their fundamental structure to their diverse functions in nature and medicine, we’ll uncover the secrets behind these remarkable molecules. Prepare to embark on a journey into the captivating realm of peptides!
Peptides are essentially small proteins, often acting as signaling molecules or components of larger protein structures. Their relatively short length, compared to proteins, allows for greater flexibility and diverse functions. Understanding their formation, properties, and applications is key to appreciating their significance in biology and medicine.
Defining Peptides
Peptides are short chains of amino acids, playing crucial roles in various biological processes. Understanding their structure and function is fundamental to comprehending the intricate workings of life. They differ from proteins, which are typically longer chains of amino acids with more complex structures and functions. This section will delve into the specifics of peptide definition, structure, naming, and examples.
Peptide Definition
A peptide is a short chain of amino acids linked together by peptide bonds. These bonds form between the carboxyl group of one amino acid and the amino group of another, resulting in the elimination of a water molecule. This process is known as dehydration synthesis. Peptides are typically composed of fewer than 50 amino acids, whereas proteins are longer chains.
Difference Between Peptides and Proteins
The primary difference between peptides and proteins lies in their length. Peptides are shorter chains of amino acids, while proteins are significantly longer. This difference in length directly impacts their structural complexity and consequently, their functional diversity. Proteins often fold into complex three-dimensional structures, enabling them to perform a wider array of tasks within biological systems. Peptides, being shorter, tend to have more localized, specific functions.
General Structure of a Peptide
A peptide’s structure involves a linear arrangement of amino acids. Each amino acid consists of a central carbon atom (alpha carbon) bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R group). The sequence of these amino acids, determined by the genetic code, dictates the peptide’s unique properties and function.
The peptide bonds connecting the amino acids are amide linkages.
Peptide bond: A covalent bond formed between the carboxyl group of one amino acid and the amino group of another.
So, what exactly is a peptide? It’s basically a short chain of amino acids, the building blocks of proteins. While peptides play a vital role in various bodily functions, did you know that certain essential oils, like lavender and peppermint, are also used to soothe headaches and migraines? For example, exploring the benefits of lavender and peppermint essential oils for relieving headaches and migraines can be really interesting.
lavender and peppermint essential oils for headaches and migraines could offer some insights into this. Regardless of the use case, understanding the basic structure of peptides is still crucial for understanding how our bodies function.
Naming Conventions for Peptides
Peptide names typically indicate the sequence of amino acids. The names are written using the three-letter abbreviations for the amino acids, in the order they appear in the chain. For example, Gly-Ala-Ser denotes a peptide containing glycine, alanine, and serine in that order. Sometimes, the one-letter abbreviations are used, for instance, Gly-Ala-Ser could also be written as G-A-S.
Examples of Common Peptides
Peptides play a wide range of roles in biological systems. Here are some examples of common peptides and their functions.
| Peptide Name | Source | Function |
|---|---|---|
| Oxytocin | Posterior pituitary gland | Stimulates uterine contractions during childbirth and milk ejection during breastfeeding. |
| Vasopressin | Posterior pituitary gland | Regulates water reabsorption in the kidneys. |
| Glucagon | Pancreas | Raises blood glucose levels. |
| Insulin | Pancreas | Lowers blood glucose levels. |
| Bradykinin | Blood | Causes vasodilation and pain. |
| Substance P | Nervous system | Transmits pain signals. |
| Enkephalins | Brain | Act as natural painkillers. |
Peptide Formation
Peptides, those essential building blocks of proteins, are formed through a fascinating process called peptide bond formation. This crucial step links amino acids together, creating the intricate structures that underpin biological functions. Understanding this process is key to appreciating the complexity and versatility of proteins.The peptide bond formation is a dehydration reaction, a process where a molecule of water is eliminated to join two molecules.
In this case, the carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH2) of another amino acid. This reaction results in the formation of a peptide bond (–CONH–) between the two amino acids and the release of a water molecule.
Peptide Bond Formation Mechanism
The formation of a peptide bond involves a nucleophilic attack. The nitrogen atom of the amino group acts as a nucleophile, attacking the carbonyl carbon of the carboxyl group. This attack leads to the formation of a tetrahedral intermediate. The tetrahedral intermediate then collapses, expelling a water molecule and creating the peptide bond. This process is facilitated by enzymes, crucial players in the peptide synthesis within living organisms.
Role of Enzymes in Peptide Synthesis, What is a peptide
Enzymes play a critical role in accelerating and directing peptide bond formation within living systems. They provide a highly controlled environment, precisely positioning the reactants and lowering the activation energy required for the reaction. This precise control ensures that peptide bonds are formed in the correct sequence, leading to the production of the specific protein needed. Without enzymes, these reactions would occur at a negligible rate, hindering the construction of complex proteins.
Laboratory Peptide Synthesis Methods
Various methods exist to synthesize peptides in a laboratory setting, each with its advantages and disadvantages. These methods range from simple to complex, depending on the desired peptide length and complexity. The choice of method is dictated by the factors like desired length, purity, and the availability of reagents.
- Solid-phase peptide synthesis (SPPS): This method is commonly used for the synthesis of longer peptides. It involves attaching one amino acid at a time to a solid support, allowing for the stepwise addition of amino acids in a predefined sequence. This technique offers a high degree of control over the reaction and allows for the purification of the product at each step, enhancing the efficiency of the process.
The solid support typically consists of a resin bead to which the growing peptide chain is attached.
- Solution-phase peptide synthesis: This method is typically employed for smaller peptides. In this method, amino acids are linked together in solution. This method, though simpler than SPPS, may lead to lower yields and increased purification challenges. Solution-phase synthesis often relies on protecting groups to prevent unwanted side reactions. Careful control of reaction conditions is critical.
So, what exactly is a peptide? Basically, it’s a short chain of amino acids, the building blocks of proteins. Interestingly, peptides play a crucial role in various bodily functions, including the complex process of continuous glucose monitoring, like with the new Dexcom 5 continuous glucose monitoring the arrival of Dexcom 5. Understanding these tiny chains helps us appreciate the intricate ways our bodies work, and how advancements like this new technology are impacting healthcare.
Importance of Reaction Conditions in Peptide Synthesis
Precise reaction conditions are paramount for successful peptide synthesis. Factors such as pH, temperature, and the presence of protecting groups directly influence the outcome of the reaction. These conditions must be carefully controlled to prevent unwanted side reactions and ensure the formation of the desired peptide.
- Protecting groups: Protecting groups are essential in preventing unwanted side reactions. These groups block specific functional groups on amino acids, protecting them from unwanted reactions during the synthesis process. These groups are then removed after the peptide bond formation is complete. This is critical to ensuring the desired peptide sequence is produced.
- Coupling reagents: Coupling reagents facilitate the formation of the peptide bond. Different coupling reagents have varying degrees of efficiency and compatibility with different amino acids. The selection of an appropriate coupling reagent is crucial to optimize peptide synthesis.
Comparison of Peptide Synthesis Methods
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Solid-phase peptide synthesis (SPPS) | Amino acids added sequentially to a solid support | High control, stepwise purification, suitable for longer peptides | Can be complex, equipment requirements, potentially lower yields for very short peptides |
| Solution-phase peptide synthesis | Amino acids linked in solution | Simple setup, potentially higher yields for very short peptides | Lower control, purification challenges, difficult for longer peptides |
Peptide Properties: What Is A Peptide
Peptides, the building blocks of proteins, exhibit a fascinating array of physical and chemical properties. Understanding these properties is crucial for comprehending their diverse roles in biological systems and their potential applications in various fields, including medicine and materials science. Their behavior is dictated by the unique combination of their amino acid sequence, the interactions between amino acids, and the surrounding environment.These properties significantly impact the function of peptides.
For instance, the solubility of a peptide directly influences its ability to interact with other molecules in solution. The stability of a peptide determines its ability to maintain its structure and function under various conditions, while the melting point reveals the energy required to disrupt its secondary and tertiary structures. These characteristics are not uniform across all peptides; they are highly dependent on the specific sequence and the chemical environment.
Solubility
Peptide solubility is a crucial factor determining their behavior in biological systems. The solubility of a peptide is largely dictated by the hydrophilic and hydrophobic amino acid residues present in its sequence. Hydrophilic residues, such as serine, threonine, and asparagine, tend to interact favorably with water molecules, increasing the peptide’s solubility. Conversely, hydrophobic residues, such as leucine, isoleucine, and valine, tend to cluster together, minimizing their interaction with water and decreasing solubility.
The overall balance of these residues influences the peptide’s ability to dissolve in various solvents. For example, a peptide rich in polar amino acids will likely be more soluble in water than a peptide predominantly composed of non-polar amino acids.
Melting Point
The melting point of a peptide represents the temperature at which the peptide transitions from an ordered, folded state to a disordered, unfolded state. This transition is often accompanied by significant changes in the peptide’s physical properties, such as solubility and activity. The precise melting point is influenced by the specific amino acid sequence, the strength of the intermolecular forces holding the peptide in its folded conformation, and the presence of any stabilizing factors like metal ions or other molecules.
Factors like hydrogen bonding, hydrophobic interactions, and disulfide bonds significantly impact the stability of the folded structure and, consequently, the melting point. Higher melting points often indicate greater stability and a more robust structure.
Stability
Peptide stability is a critical characteristic that determines their ability to maintain their structure and function under various conditions. This stability is dependent on the interplay of various factors. For example, the presence of disulfide bonds significantly enhances the stability of a peptide by creating strong covalent linkages between cysteine residues. These covalent bonds help to maintain the peptide’s folded structure, even in harsh conditions.
The surrounding environment also plays a critical role. High temperatures, extreme pH values, and the presence of proteolytic enzymes can all negatively impact peptide stability. Furthermore, the presence of stabilizing elements, such as metal ions, can contribute to the overall stability of the peptide.
Factors Influencing Folding and Conformation
Peptide folding and conformation are crucial for determining their function. The specific amino acid sequence dictates the potential folding patterns, and the surrounding environment can influence these patterns. Hydrogen bonding, hydrophobic interactions, and electrostatic interactions between amino acid residues drive the folding process. The precise arrangement of these interactions shapes the unique three-dimensional structure of the peptide, which is crucial for its function.
Specific examples include alpha-helices, beta-sheets, and random coils, each with different properties and functions. External factors such as temperature, pH, and the presence of other molecules can also alter the peptide’s conformation, influencing its activity.
Peptide Sequence and Properties
The amino acid sequence of a peptide directly dictates its physical and chemical properties. A change in even a single amino acid can significantly alter the peptide’s solubility, stability, and overall function. For instance, replacing a hydrophilic amino acid with a hydrophobic one can drastically reduce the peptide’s water solubility. Similarly, alterations in the sequence can affect the formation of secondary structures, impacting the peptide’s stability and overall conformation.
This highlights the profound impact of sequence on function.
Comparison of Peptide Properties
| Peptide Type | Solubility | Melting Point | Stability |
|---|---|---|---|
| Short, hydrophilic peptides | High | Low | Low |
| Long, hydrophobic peptides | Low | High | High |
| Cyclized peptides | Variable | High | High |
| Peptides with disulfide bonds | Variable | High | High |
Peptide Functions
Peptides, those short chains of amino acids, are not just building blocks; they are active players in a multitude of biological processes. Their diverse functions stem from their unique structures and the specific arrangements of amino acids within their sequences. Understanding these functions is crucial to appreciating the intricate workings of life itself.
Examples of Peptide Functions in Biological Systems
Peptides play diverse roles in biological systems, from coordinating cellular responses to catalyzing biochemical reactions. Their specific functions depend on the unique sequence and structure of the peptide. Some peptides act as hormones, regulating physiological processes, while others act as neurotransmitters, transmitting signals between nerve cells. Still others are integral components of the immune system, defending the body against pathogens.
- Hormones: Many hormones are peptides, such as insulin, glucagon, and growth hormone. These peptides regulate various metabolic processes, including blood sugar levels and growth. Insulin, for example, lowers blood glucose levels by promoting glucose uptake into cells. Glucagon, on the other hand, raises blood glucose levels by stimulating the release of glucose from the liver.
- Neurotransmitters: Some peptides act as neurotransmitters, carrying signals between nerve cells. Examples include substance P, involved in pain transmission, and endorphins, which act as natural pain relievers. These peptides play critical roles in modulating neuronal activity and influencing behavior.
- Immune System Peptides: The immune system employs peptides to defend against pathogens. Cytokines, a diverse group of signaling peptides, regulate immune responses. Interleukins and interferons are examples of cytokines that orchestrate the inflammatory response and antiviral defenses.
Roles of Peptides in Signaling Pathways
Peptides are essential components in signaling pathways, mediating communication between cells. These peptides act as ligands, binding to specific receptors on target cells, triggering intracellular cascades that lead to various cellular responses. The specific sequence of amino acids dictates the type of signal transmitted and the cellular response.
- Receptor Binding: Peptides bind to specific receptors on the surface of target cells. This binding initiates a cascade of intracellular events. The interaction between the peptide and the receptor is highly specific, ensuring the precise delivery of the signal.
- Intracellular Signaling: Upon binding, the receptor undergoes conformational changes, initiating a series of events within the cell. These intracellular signaling cascades involve a complex interplay of proteins, enzymes, and second messengers, ultimately leading to the desired cellular response. The specificity of the peptide-receptor interaction ensures the precise regulation of cellular processes.
Role of Peptides in Enzyme Activity
Peptides can influence enzyme activity in various ways. Some peptides act as inhibitors, blocking enzyme function, while others act as activators, enhancing enzyme activity. These peptides can bind to the active site of the enzyme, directly affecting its catalytic ability.
Peptides are essentially short chains of amino acids, playing crucial roles in various bodily functions. Understanding these small molecules is fascinating, especially when considering how they might be related to conditions like premenstrual dysphoria. Recent research is exploring the potential for innovative therapies like surgical management of premenstrual dysphoria disorder , which could impact the hormonal imbalances that contribute to this condition.
Ultimately, a deeper understanding of peptides is key to unraveling these complexities and developing effective treatments.
- Enzyme Inhibition: Certain peptides act as potent inhibitors of specific enzymes. These peptides bind to the active site of the enzyme, preventing substrate binding and hindering the catalytic reaction. This inhibitory mechanism is crucial in regulating enzyme activity and preventing unwanted biochemical processes.
- Enzyme Activation: Other peptides act as activators, enhancing the activity of specific enzymes. These peptides bind to the enzyme, altering its conformation and increasing its catalytic efficiency. This activation process can be essential in regulating various metabolic pathways.
Examples of Peptides Used in Medicine and Pharmaceuticals
Peptides find widespread applications in medicine and pharmaceuticals. Their ability to target specific receptors and regulate cellular processes makes them valuable tools for treating various diseases. Examples include various therapeutic peptides used in treating diabetes, hypertension, and other conditions.
- Therapeutic Peptides: Many peptides are used as therapeutic agents in medicine. Examples include insulin, used in treating diabetes, and various growth factors used in wound healing and tissue regeneration. These peptides are administered to modulate specific physiological processes, thereby mitigating the effects of diseases.
Classification of Peptides Based on Biological Functions
| Peptide Function | Examples | Mechanism |
|---|---|---|
| Hormones | Insulin, Glucagon, Growth Hormone | Regulate metabolic processes |
| Neurotransmitters | Substance P, Endorphins | Transmit signals between nerve cells |
| Immune System Regulators | Cytokines (Interleukins, Interferons) | Modulate immune responses |
| Enzyme Inhibitors/Activators | Specific peptide inhibitors/activators | Regulate enzyme activity |
Peptide Analysis
Unraveling the secrets of peptides often hinges on meticulous analysis. Understanding their composition, sequence, and presence within complex mixtures is crucial for research in diverse fields, from drug discovery to clinical diagnostics. This crucial step enables researchers to identify and quantify peptides, providing insights into their biological roles and functions. Detailed analysis allows for a deeper understanding of peptide behavior and their interactions within biological systems.Precise determination of a peptide’s sequence is paramount.
Various sophisticated techniques have been developed for this purpose, offering high resolution and accuracy. Effective identification of peptides within complex mixtures also presents a significant challenge. A wide array of analytical tools are employed to separate and characterize these molecules, ensuring accurate and reliable results. The importance of this analytical process in various research areas and clinical applications is undeniable.
Determining Peptide Sequence
Determining the sequence of a peptide is a fundamental aspect of peptide analysis. Several powerful methods are available, each with its own strengths and limitations. These methods are crucial for elucidating the structure-function relationships of peptides and for validating their predicted function.
- Edman Degradation: This technique is a classic method for sequencing peptides. It sequentially removes amino acid residues from the N-terminus of the peptide, allowing for the identification of each amino acid in the chain. The process involves chemical modification and cleavage of the N-terminal amino acid, followed by its identification through various chromatographic techniques. This method is particularly useful for smaller peptides.
However, it is less suitable for very large peptides or those with repetitive sequences.
- Mass Spectrometry: Mass spectrometry (MS) is a highly versatile technique for peptide sequencing. It measures the mass-to-charge ratio of ionized peptides. By fragmenting the peptide ions, MS provides information about the amino acid sequence. Different types of MS experiments, such as tandem mass spectrometry (MS/MS), are particularly effective in determining the sequence of peptides. Sophisticated software algorithms analyze the fragmentation patterns to deduce the amino acid sequence.
MS is widely used for sequencing peptides from complex mixtures, making it a powerful tool for proteomics research.
Identifying Peptides in Complex Mixtures
Analyzing peptides in complex biological samples, such as blood serum or tissue extracts, is a significant challenge. Sophisticated separation techniques are essential to isolate individual peptides before their characterization. The use of these methods allows researchers to understand the role of peptides in various biological processes.
- High-Performance Liquid Chromatography (HPLC): HPLC is a widely used technique for separating peptides based on their different properties, such as hydrophobicity or charge. The separation is achieved by passing the peptide mixture through a column packed with a stationary phase. Different peptides will elute at different times, allowing for their isolation and subsequent analysis by MS. This technique is particularly useful when dealing with mixtures containing peptides with similar properties.
It allows for the separation and isolation of individual peptides from complex mixtures.
- Capillary Electrophoresis (CE): CE is another powerful separation technique that utilizes an electric field to separate charged molecules based on their size and charge. It offers high resolution and speed compared to HPLC. CE is particularly advantageous when dealing with peptides that are difficult to separate by HPLC. It is commonly used in conjunction with MS for peptide identification and quantification in complex biological samples.
Common Analytical Tools
Various analytical tools are employed in peptide analysis, each offering unique capabilities. These tools are essential for researchers to gain a comprehensive understanding of peptide behavior and their roles in biological systems.
- Mass Spectrometers: These instruments are crucial for determining the mass-to-charge ratio of ionized peptides, leading to sequence elucidation and quantification. They play a vital role in proteomics and peptide-based diagnostics.
- Chromatographs (HPLC and CE): These instruments enable the separation of peptides based on their physical and chemical properties. They are crucial for isolating individual peptides for detailed analysis by MS.
Importance of Peptide Analysis
Peptide analysis is crucial in various research and clinical settings. It plays a significant role in drug discovery, diagnostics, and understanding fundamental biological processes. Analyzing peptides allows researchers to investigate their function and interactions with other molecules.
| Method | Principle | Applications |
|---|---|---|
| Edman Degradation | Sequential removal of N-terminal amino acids | Sequencing smaller peptides |
| Mass Spectrometry (MS) | Measurement of mass-to-charge ratio of ionized peptides | Sequencing peptides in complex mixtures, proteomics |
| High-Performance Liquid Chromatography (HPLC) | Separation of peptides based on properties like hydrophobicity or charge | Isolating peptides from complex mixtures |
| Capillary Electrophoresis (CE) | Separation of charged molecules based on size and charge | High-resolution separation of peptides, particularly those difficult to separate by HPLC |
Peptide Applications
Peptides, those short chains of amino acids, are proving to be remarkably versatile molecules with a wide range of applications. Their specific structures and properties allow them to interact with various biological systems and materials, leading to exciting possibilities in diverse industries. From enhancing food products to revolutionizing personal care, peptides are making significant strides in various fields.
Peptide Applications in Food Science
Peptides contribute significantly to the advancement of food science. Their ability to influence taste, texture, and nutritional value makes them valuable ingredients. Hydrolyzed proteins, rich in bioactive peptides, can improve the nutritional profile of foods, potentially increasing the bioavailability of essential nutrients. Furthermore, certain peptides exhibit antimicrobial or antioxidant properties, enhancing the shelf life and safety of food products.
The use of specific peptides can even modify the mouthfeel of foods, contributing to a more enjoyable eating experience.
Peptide Applications in Agriculture
Peptides are finding applications in agricultural practices as well. Their use as biostimulants and plant growth regulators has shown promise. Specific peptides can promote plant development by enhancing nutrient uptake or improving stress tolerance. Additionally, some peptides exhibit insecticidal or fungicidal properties, offering sustainable alternatives to traditional chemical pesticides. This environmentally friendly approach to pest control could have a positive impact on agricultural practices.
Peptide Applications in Cosmetics and Personal Care
Peptides are increasingly incorporated into cosmetics and personal care products. Their ability to stimulate collagen production or improve skin elasticity makes them popular ingredients in anti-aging products. Certain peptides may also possess antioxidant properties, helping to protect the skin from environmental damage. Moreover, some peptides can promote wound healing or reduce inflammation, expanding their application in various personal care products.
Peptide Applications in Materials Science
Peptides are demonstrating potential in materials science. Their unique structural features can be exploited to create novel materials with tailored properties. Specific peptide sequences can self-assemble into ordered structures, leading to the development of bio-inspired materials with enhanced strength, flexibility, or biocompatibility. This opens doors to applications in areas like tissue engineering and biocompatible coatings.
Table of Peptide Applications in Different Industries
| Industry | Application | Example |
|---|---|---|
| Food Science | Enhancing flavor, texture, nutritional value, and shelf life | Hydrolyzed whey protein peptides improving protein digestibility and increasing the nutritional value of dairy products. |
| Agriculture | Biostimulants, plant growth regulators, pest control | Peptides promoting plant growth and stress tolerance, or inhibiting fungal infections. |
| Cosmetics & Personal Care | Anti-aging, skin elasticity, wound healing, reducing inflammation | Peptides stimulating collagen production in anti-aging creams, or promoting wound healing in topical treatments. |
| Materials Science | Bio-inspired materials with tailored properties | Peptides self-assembling into nanofibers for tissue engineering scaffolds or biocompatible coatings for implants. |
Illustrative Examples
Peptides, the humble building blocks of proteins, play a vast array of roles in biological systems. From orchestrating intricate signaling pathways to forming structural components, peptides’ diverse functions are crucial for life. Understanding specific examples illuminates the breadth of their impact. This section delves into illustrative cases, highlighting naturally occurring peptides and their synthetic counterparts.Exploring concrete examples of peptides offers a tangible grasp of their diverse roles.
The structural intricacies and biological functions of peptides, both natural and synthetic, provide valuable insights into their significance.
Naturally Occurring Peptides with Biological Roles
Peptides are abundant in nature, each with specialized functions. Understanding their structural properties and biological roles unveils the intricate workings of biological systems. These naturally occurring peptides are often involved in crucial processes, including hormone regulation, neurotransmission, and immune responses.
- Oxytocin (OT): A neuropeptide with nine amino acids, oxytocin is synthesized in the hypothalamus and plays a vital role in social bonding and reproduction. It stimulates uterine contractions during childbirth and milk ejection during breastfeeding. Oxytocin’s effects extend beyond these physiological processes, influencing social behaviors like trust and empathy. It’s a crucial hormone for maternal care and social interactions.
- Vasopressin (AVP): Another crucial neuropeptide, vasopressin, is also synthesized in the hypothalamus. Similar to oxytocin, it plays a significant role in regulating social behaviors. Vasopressin influences blood pressure regulation, water retention, and stress responses. Its actions are critical for maintaining homeostasis.
- Enkephalins: These opioid peptides, composed of five or six amino acids, act as natural pain relievers in the central nervous system. They bind to opioid receptors, modulating pain signals and influencing mood. Enkephalins’ role in pain management and their connection to emotional states make them important for understanding these complex processes.
Synthetic Peptides in Research and Drug Development
Synthetic peptides have become indispensable tools in research and drug development. Their precise structure allows for targeted investigation of specific biological processes. Researchers can manipulate amino acid sequences to explore peptide function and design novel therapeutics.
- Angiotensin II: A crucial peptide involved in blood pressure regulation. Synthetic analogs of angiotensin II are frequently used in research to study the effects of different sequences on blood pressure and cardiovascular function. This aids in the development of potential treatments for hypertension.
- Growth hormone-releasing hormone (GHRH): Synthetic peptides mimic natural hormones and have applications in various medical conditions. For example, GHRH analogs are used to stimulate growth hormone secretion in specific cases, such as in children with growth hormone deficiency. This highlights the therapeutic potential of peptide-based therapies.
A Detailed Description of a Specific Peptide
Gramicidin S is a cyclic decapeptide with a unique structure. Its hydrophobic amino acid residues create a channel across cell membranes. This unique property allows it to permeabilize cell membranes, disrupting cellular function. This characteristic makes it an intriguing subject for research, potentially useful in antimicrobial applications.
- Gramicidin S: A cyclic decapeptide, gramicidin S, possesses a unique structure that enables it to form channels across cell membranes. This property makes it a promising candidate for antimicrobial applications. The hydrophobic amino acid residues in its structure create a pathway through the cell membrane, disrupting cellular function and potentially leading to bacterial cell death. Its structure and mechanism of action offer a fascinating insight into the intricate interactions between peptides and cell membranes.
Last Word

In summary, peptides are multifaceted molecules with profound biological significance. From their simple yet intricate structures to their varied roles in signaling, enzyme activity, and medicine, they are crucial to life as we know it. This exploration has highlighted the importance of understanding peptide formation, properties, analysis, and applications across various industries. Further research promises to unveil even more of the mysteries hidden within these essential building blocks.