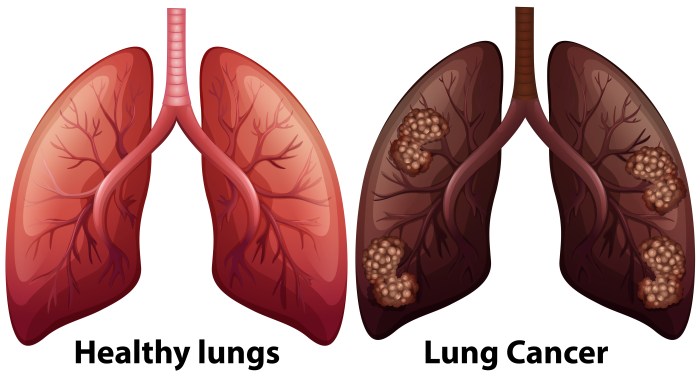
Metastatic adenocarcinoma overview and more delves into the complexities of this aggressive cancer. We’ll explore its defining characteristics, the often-devastating journey of diagnosis and treatment, and the vital support systems available for patients and families. From understanding the different stages to examining treatment options and prognosis, this comprehensive guide aims to equip readers with a clearer understanding of metastatic adenocarcinoma.
This detailed look at metastatic adenocarcinoma will cover the key aspects of the disease, including its causes, risk factors, diagnosis, treatment options, prognosis, and patient support. We’ll present information in an organized and accessible manner, incorporating tables and case studies to enhance comprehension and illustrate real-world experiences.
Introduction to Metastatic Adenocarcinoma
Metastatic adenocarcinoma is a severe form of cancer characterized by the uncontrolled spread of cancerous cells originating from glands. These cells, initially growing within a gland-like structure (adenocarcinoma), break free and travel through the bloodstream or lymphatic system to establish new tumors in distant organs. This process, metastasis, dramatically impacts the patient’s prognosis and treatment options.
Defining Metastatic Adenocarcinoma
Metastatic adenocarcinoma is a type of cancer where malignant cells originating from glandular tissue (adenocarcinoma) have spread to other parts of the body. This spread, or metastasis, is a key distinction from the original, localized adenocarcinoma. The defining characteristic is the presence of cancerous cells outside the primary tumor site, which indicates a more advanced and aggressive disease stage.
Diving into metastatic adenocarcinoma overview and more reveals a complex disease. While researching, I stumbled upon some fascinating insights into the relationship between aging and other conditions, like lupus. For example, did you know that certain factors related to aging can affect lupus symptoms? Learning about these connections, as detailed in this helpful article on five facts about lupus and aging , can offer valuable context when exploring the overall landscape of metastatic adenocarcinoma.
This deeper understanding is key to comprehending the various factors influencing this type of cancer.
Key Characteristics Distinguishing It from Other Cancers
Metastatic adenocarcinoma, unlike other cancers, exhibits a unique cellular structure and growth pattern stemming from its glandular origin. Its ability to spread to distant organs, a hallmark of advanced disease, is a key differentiator. Genetic mutations and epigenetic alterations drive the development and progression of metastatic adenocarcinoma, leading to a diverse range of clinical presentations.
Learning about metastatic adenocarcinoma is crucial for understanding its progression. While exploring different lifestyle choices, like whether vaping affects weight loss, can be interesting, it’s important to remember that these factors don’t directly address the core issues of the disease. For a deeper dive into the complexities of metastatic adenocarcinoma and related treatments, check out this insightful article on does vaping make you lose weight.
Ultimately, focusing on accurate medical information about metastatic adenocarcinoma is key.
Stages of Metastatic Adenocarcinoma
Staging of metastatic adenocarcinoma is crucial for determining the extent of the disease and guiding treatment decisions. Staging systems, such as the TNM system, categorize the tumor size (T), lymph node involvement (N), and presence of distant metastasis (M). The higher the stage, the more extensive the spread of the cancer, indicating a less favorable prognosis.
Common Sites of Metastasis
Metastatic adenocarcinoma frequently spreads to specific organs due to factors such as blood supply, organ structure, and cellular affinity. Common sites of metastasis include the lungs, liver, brain, bones, and peritoneum. The specific sites of metastasis can vary depending on the primary tumor’s location and the individual patient’s characteristics.
Types and Symptoms of Metastatic Adenocarcinoma
| Type of Metastatic Adenocarcinoma | Common Symptoms |
|---|---|
| Metastatic lung adenocarcinoma | Cough, shortness of breath, chest pain, fatigue, weight loss |
| Metastatic breast adenocarcinoma | Lump or thickening in the breast, skin dimpling or changes, nipple discharge, bone pain, fatigue |
| Metastatic colorectal adenocarcinoma | Abdominal pain, blood in stool, change in bowel habits, fatigue, weight loss |
| Metastatic pancreatic adenocarcinoma | Abdominal pain, jaundice, weight loss, nausea, vomiting |
| Metastatic ovarian adenocarcinoma | Abdominal bloating, pelvic pain, abdominal distension, fatigue, weight loss |
The symptoms listed in the table are not exhaustive and may vary depending on the specific site of metastasis and the individual patient. Consulting a healthcare professional is essential for accurate diagnosis and personalized treatment.
Causes and Risk Factors
Unraveling the mysteries behind metastatic adenocarcinoma requires understanding the complex interplay of genetic predispositions, environmental exposures, and lifestyle choices. While a definitive cause is often elusive, research consistently points towards a confluence of factors that increase an individual’s susceptibility. Pinpointing these factors is crucial for developing targeted prevention strategies and personalized treatment plans.
Potential Causes
Metastatic adenocarcinoma, a devastating form of cancer, doesn’t have a single, identifiable cause. Instead, it’s thought to stem from a combination of factors, including genetic mutations and environmental exposures. These factors interact in intricate ways, leading to the uncontrolled growth and spread of cancerous cells. Understanding these factors is essential to develop preventive measures and personalized treatment strategies.
Genetic Predispositions
Certain inherited genetic mutations significantly increase the risk of developing metastatic adenocarcinoma. These mutations can affect genes involved in DNA repair, cell growth, and development. Examples include mutations in BRCA1 and BRCA2 genes, which are associated with a higher risk of various cancers, including breast and ovarian cancers, and in some cases, a heightened risk of metastatic adenocarcinoma.
Family history of similar cancers is a strong indicator of potential genetic predisposition. Individuals with a family history of these cancers should consider genetic testing to assess their risk.
Environmental Factors, Metastatic adenocarcinoma overview and more
Environmental exposures play a vital role in the development of metastatic adenocarcinoma. Exposure to certain carcinogens, such as asbestos, certain industrial chemicals, and prolonged exposure to radiation, can damage DNA and increase the likelihood of cancer development. Furthermore, dietary factors, lifestyle choices, and chronic inflammation are also suspected environmental factors. It’s crucial to emphasize that the interaction between genetics and environment is key to understanding the risk of developing metastatic adenocarcinoma.
Environmental factors can trigger or accelerate the progression of pre-existing genetic mutations.
Comparison of Risk Factors Across Subtypes
Different subtypes of metastatic adenocarcinoma can have varying risk profiles. For instance, lung adenocarcinoma, a common subtype, may be linked to smoking and exposure to air pollution. Similarly, colorectal adenocarcinoma often correlates with dietary factors, obesity, and inflammatory bowel disease. These variations in risk factors highlight the need for a nuanced approach to prevention and treatment tailored to specific subtypes.
Summary of Risk Factors
| Risk Factor | Description | Evidence |
|---|---|---|
| Genetic Predisposition | Inherited mutations in genes like BRCA1/2. | Strong correlation observed in family studies and genetic testing. |
| Environmental Exposures | Exposure to carcinogens (e.g., asbestos, industrial chemicals), radiation. | Epidemiological studies show an association between exposure and increased risk. |
| Lifestyle Choices | Smoking, diet, obesity, lack of physical activity. | Studies suggest correlations between these factors and cancer development. |
| Chronic Inflammation | Long-term inflammation in the body. | Evidence shows chronic inflammation can promote cancer growth. |
| Specific Dietary Factors | High fat diets, processed foods, lack of fruits and vegetables. | Correlation found in studies of dietary habits and cancer incidence. |
| Age | Increased risk with advancing age. | Observational studies show a trend of higher incidence with age. |
Diagnosis and Testing
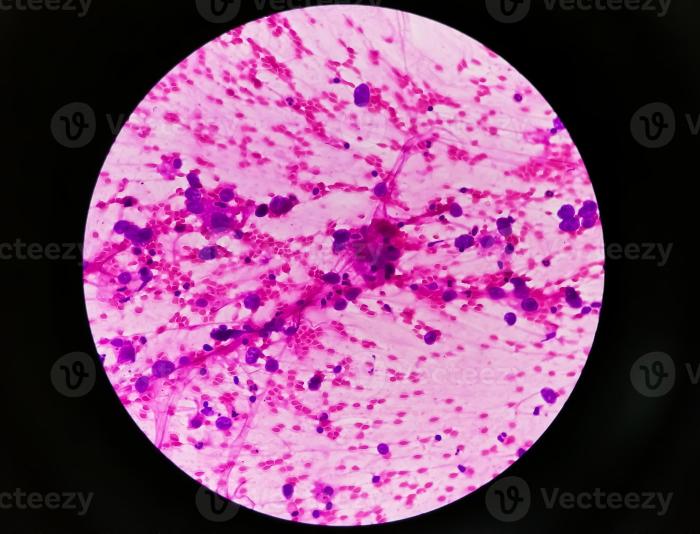
Unveiling the presence of metastatic adenocarcinoma requires a meticulous approach, combining various diagnostic tools. Accurate identification is crucial for tailoring treatment plans and maximizing patient outcomes. The process often involves a series of tests, each contributing to a comprehensive picture of the disease.Early detection significantly improves the chances of successful treatment. A multi-faceted approach, incorporating imaging, laboratory analysis, and biopsy procedures, is essential for precise diagnosis.
This allows clinicians to understand the extent of the cancer’s spread and guide subsequent therapeutic strategies.
Imaging Techniques
Imaging plays a pivotal role in identifying metastatic adenocarcinoma. Different imaging modalities offer varying degrees of detail and sensitivity. These techniques help visualize the extent of the disease, locating primary tumors and secondary sites of spread. The specific imaging techniques employed depend on the suspected location of the cancer and its potential spread.
- Computed Tomography (CT) Scans: CT scans use X-rays and computer processing to create detailed cross-sectional images of the body. They are valuable for assessing the extent of the cancer’s spread within the chest, abdomen, and pelvis. CT scans are widely used to visualize potential sites of metastasis, including the lungs, liver, and bones.
- Magnetic Resonance Imaging (MRI) Scans: MRI scans utilize powerful magnetic fields and radio waves to generate detailed images of internal structures. They are often used to visualize the brain and spinal cord, providing detailed images of soft tissues. This is particularly helpful in assessing potential brain metastases, a serious complication.
- Positron Emission Tomography (PET) Scans: PET scans combine imaging with biological function. A radioactive tracer is administered, and the scan detects areas of increased metabolic activity. Areas of high metabolic activity, often associated with tumors, show up brightly on the scan. This technique is particularly helpful in identifying sites of metastasis that may not be apparent on other imaging modalities.
Laboratory Tests
A battery of laboratory tests is essential to complement imaging studies. These tests help assess the patient’s overall health, identify markers associated with cancer, and monitor the effectiveness of treatment.
- Blood Tests: Complete blood counts (CBCs) and comprehensive metabolic panels (CMPs) provide information about the patient’s blood cell counts, electrolytes, and organ function. These tests are crucial for evaluating the patient’s overall health and potential complications. Elevated tumor markers (like CA-125) may be indicative of metastatic adenocarcinoma, but they are not definitive on their own.
- Tumor Markers: Some specific proteins, called tumor markers, are often elevated in individuals with certain types of cancer. While not always present, these markers can offer clues about the presence and extent of the disease. However, they must be interpreted in conjunction with other diagnostic findings.
Biopsy
A definitive diagnosis often requires a biopsy. A biopsy involves the removal of a tissue sample for microscopic examination. This process helps confirm the presence of cancer cells, determine the type of cancer, and assess the grade of malignancy.
- Types of Biopsies: Various biopsy techniques are available, including fine-needle aspiration, core needle biopsy, and surgical biopsy. The choice of biopsy method depends on the location and accessibility of the suspicious area. For example, a fine-needle aspiration is less invasive than a surgical biopsy, making it a suitable option for certain sites.
- Pathology Report: A pathologist examines the tissue sample under a microscope. The pathologist’s report provides crucial information, including the type of cancer cells present, the degree of differentiation, and the presence of any other relevant features. This information is essential for treatment planning.
Diagnostic Tests Summary
| Test | Procedure | Typical Findings |
|---|---|---|
| CT Scan | X-ray imaging with contrast | Highlights anatomical structures, detects abnormalities in soft tissues |
| MRI Scan | Magnetic fields and radio waves | Detailed visualization of soft tissues, useful for brain and spinal cord |
| PET Scan | Radioactive tracer | Highlights areas of increased metabolic activity, useful for identifying metastases |
| Blood Tests | Measures various blood components | Evaluates overall health, identifies potential markers |
| Biopsy | Tissue sample removal | Confirms cancer presence, type, and grade |
Treatment Options
Navigating the complexities of metastatic adenocarcinoma requires a multifaceted approach, tailoring treatment to the specific characteristics of the cancer and the patient’s overall health. Different treatment strategies may be used alone or in combination, and the best course of action is determined by a multidisciplinary team of medical professionals. The goal is to effectively control the disease, manage symptoms, and improve quality of life.
Common Treatment Approaches
Treatment for metastatic adenocarcinoma typically involves a combination of approaches, rather than a single cure-all. This approach often targets both the primary tumor and the spread to other organs. Surgery, chemotherapy, targeted therapies, and radiation therapy are commonly used, with the specific combination chosen based on the patient’s individual circumstances.
Chemotherapy
Chemotherapy drugs are often employed in metastatic adenocarcinoma to target rapidly dividing cancer cells. These drugs work by either killing the cancer cells directly or preventing their growth and division. The choice of chemotherapy regimen depends on factors like the type of adenocarcinoma, the extent of metastasis, and the patient’s overall health. Examples of chemotherapy regimens include cisplatin, carboplatin, and paclitaxel.
Side effects can include nausea, vomiting, hair loss, and fatigue.
Targeted Therapies
Targeted therapies are increasingly used in treating metastatic adenocarcinoma. These drugs are designed to specifically target certain molecules or pathways that are involved in cancer cell growth and spread. Examples of targeted therapies include inhibitors of epidermal growth factor receptor (EGFR) or vascular endothelial growth factor receptor (VEGF). The effectiveness of targeted therapies depends on the specific genetic mutations present in the tumor.
Targeted therapies may offer a more precise approach to cancer treatment than chemotherapy, often with fewer side effects.
Surgery
Surgical intervention plays a role in the treatment of metastatic adenocarcinoma, but its application is often limited to specific circumstances. If the primary tumor is localized and surgically accessible, resection may be considered, particularly if it’s not causing significant spread. The goal of surgery is to remove the primary tumor, reducing the burden of disease and potentially slowing progression.
However, once the cancer has spread extensively, surgical resection of the primary tumor alone may not be curative. It’s important to understand that surgery is rarely the sole treatment for metastatic adenocarcinoma.
Comparison of Treatment Strategies
Different treatment strategies for metastatic adenocarcinoma offer varying degrees of effectiveness and associated side effects. Chemotherapy often achieves a significant initial response, but its effectiveness may diminish over time. Targeted therapies can provide longer-term benefits in some cases, but they are not always effective for all patients. Surgery is usually reserved for localized disease and may be combined with other treatments.
The optimal approach is determined by careful consideration of individual patient characteristics and tumor characteristics.
Treatment Options and Potential Side Effects
| Treatment Option | Potential Side Effects |
|---|---|
| Chemotherapy | Nausea, vomiting, hair loss, fatigue, mouth sores, increased risk of infection |
| Targeted Therapies | Fatigue, skin rash, diarrhea, high blood pressure, increased risk of bleeding |
| Surgery | Pain, infection, bleeding, scarring, potential for recurrence |
| Radiation Therapy | Fatigue, skin irritation, inflammation, pain, potential for long-term side effects |
Prognosis and Survival Rates: Metastatic Adenocarcinoma Overview And More
Understanding the prognosis of metastatic adenocarcinoma is crucial for patients and their families. Prognosis, essentially a prediction of the likely course and outcome of the disease, is influenced by a multitude of factors. These factors, ranging from the initial stage of the cancer to the effectiveness of treatment, play a critical role in determining the overall outlook. A clear understanding of these factors empowers informed decision-making and allows for realistic expectations.
Factors Influencing Prognosis
Several factors significantly impact the prognosis of metastatic adenocarcinoma. These factors include the specific type of adenocarcinoma, the extent of the metastasis (how far the cancer has spread), the patient’s overall health, age, and the presence of any other underlying medical conditions. The aggressiveness of the tumor itself, measured by its rate of growth and the way it responds to treatment, also plays a crucial role.
Impact of Disease Stage
The stage of the disease is a critical determinant of prognosis. Metastatic adenocarcinoma is typically staged based on the extent of the spread. Early detection and treatment are paramount for improved outcomes. More advanced stages, where the cancer has spread extensively, often have a less favorable prognosis. Staging systems provide a standardized framework for categorizing the disease’s progression and influence treatment decisions.
Typical Survival Rates
Survival rates for metastatic adenocarcinoma vary significantly depending on the stage of the disease at diagnosis. Generally, earlier stages of the disease have better survival rates compared to later stages. Survival rates are also influenced by the individual patient’s response to treatment.
Survival Rates Based on Treatment Approaches
Different treatment approaches for metastatic adenocarcinoma can impact survival rates. For instance, targeted therapies designed to attack specific cancer cells, chemotherapy regimens, and surgical interventions, when applicable, all influence the outcome. The combination of these approaches and the patient’s response to them are key factors in determining the length and quality of life. Factors such as the patient’s age, overall health, and the specific type of adenocarcinoma are also taken into account when assessing potential outcomes.
Summary Table of Survival Rates
| Stage | Approximate 5-Year Survival Rate (Example) | Explanation |
|---|---|---|
| Stage I | 40-60% | This stage usually involves localized disease, often with a good chance of cure or long-term control with treatment. |
| Stage II | 30-50% | The cancer may have spread to nearby tissues or lymph nodes. Treatment options and outcomes are influenced by the extent of spread. |
| Stage III | 20-40% | The cancer has spread to distant organs or lymph nodes. Treatment options are more complex, and outcomes are typically less favorable. |
| Stage IV (Metastatic) | 10-30% | The cancer has spread extensively throughout the body. Survival rates are generally lower, and treatment focuses on extending life and managing symptoms. |
Note: Survival rates are approximate and can vary based on individual patient factors, specific treatment approaches, and the type of adenocarcinoma. These figures are examples and should not be used for personal medical advice. Always consult with a qualified healthcare professional for personalized guidance.
Understanding metastatic adenocarcinoma is crucial, but did you know timing plays a role in other health goals? For example, optimizing your collagen intake might be beneficial, and figuring out the best time to take collagen could be important for overall well-being. This knowledge, alongside research into metastatic adenocarcinoma treatments and potential side effects, is key to making informed decisions about your health journey.
Patient Support and Resources

Navigating a metastatic adenocarcinoma diagnosis can be incredibly challenging, both physically and emotionally. Beyond the medical treatment, a robust support system is crucial for patients and their families to cope with the emotional rollercoaster and practical demands of the disease. Finding reliable resources and connecting with others facing similar journeys can make a significant difference in quality of life.
Support Groups for Patients and Families
Support groups provide a safe and empathetic space for patients and their families to share experiences, receive emotional support, and learn coping strategies. These groups often consist of individuals facing similar challenges, fostering a sense of community and understanding. Sharing stories and strategies with others who truly comprehend the unique struggles of this disease can offer invaluable comfort and guidance.
Importance of Emotional Support
Emotional support plays a critical role in managing the emotional toll of metastatic adenocarcinoma. The diagnosis and treatment often trigger a wide range of emotions, including anxiety, fear, sadness, and anger. Acknowledging and addressing these emotions is vital for maintaining well-being. Seeking professional counseling or therapy can be extremely beneficial in developing healthy coping mechanisms. Support groups and counseling can help patients and families process these emotions and develop strategies to manage the stress associated with the disease.
Role of Palliative Care
Palliative care focuses on improving the quality of life for individuals facing serious illnesses. It’s not about curing the disease, but rather about managing symptoms, alleviating pain, and providing emotional support to patients and their families. Palliative care teams work closely with the medical team to develop a comprehensive care plan that addresses physical, emotional, and spiritual needs.
This holistic approach can significantly improve a patient’s overall well-being and enable them to focus on living their lives as fully as possible, despite the challenges of the disease.
Reputable Organizations Offering Support and Information
Numerous organizations offer invaluable support and information to patients with metastatic adenocarcinoma and their families. These organizations provide resources, educational materials, and connect individuals with support groups. It’s important to research and select organizations that are reputable, have a proven track record, and align with your specific needs.
- American Cancer Society (ACS): Offers comprehensive information, support groups, and resources for patients and families. They provide educational materials, emotional support, and practical guidance for navigating the challenges of cancer treatment.
- National Cancer Institute (NCI): A leading source of information on cancer research, treatment, and support. Their website provides detailed information on various cancer types, including metastatic adenocarcinoma.
- The American Society of Clinical Oncology (ASCO): Provides resources for medical professionals, but also offers patient education materials on a wide range of cancers, including metastatic adenocarcinoma.
- Local Cancer Centers: Many hospitals and cancer centers have dedicated patient support programs and resources. These programs can provide access to support groups, counseling services, and educational workshops.
Illustrative Case Studies
Understanding metastatic adenocarcinoma requires looking at real-life examples. These case studies provide insight into the diverse experiences of patients facing this challenging disease, highlighting the importance of personalized care and the impact of treatment on their lives. Each case demonstrates the complexities of the disease and the importance of tailoring treatment to individual needs.
Case Study 1: A Patient’s Journey with Metastatic Adenocarcinoma of the Lung
This case study follows a 62-year-old female patient diagnosed with metastatic adenocarcinoma originating in the lungs. Early symptoms included persistent cough, shortness of breath, and unexplained weight loss. The patient’s medical history included a 20-year smoking history, a known risk factor for lung cancer.
| Symptoms | Diagnosis | Treatment | Outcome |
|---|---|---|---|
| Persistent cough, shortness of breath, unexplained weight loss | Metastatic adenocarcinoma of the lung, confirmed by biopsy and imaging studies (CT scan, PET scan). | A multi-modal approach involving chemotherapy (e.g., platinum-based regimens), targeted therapy (e.g., EGFR inhibitors if appropriate), and radiation therapy to specific sites of metastasis. The patient also underwent supportive care for symptom management. | The patient experienced a significant improvement in quality of life during the initial treatment phases. However, the disease progressed over time, and the patient ultimately passed away after 3 years of diagnosis. The treatment helped manage symptoms and prolong survival, but the disease was ultimately too advanced to be cured. |
Personalized treatment plans are crucial in managing metastatic adenocarcinoma. The patient’s treatment involved considering factors such as the type of adenocarcinoma, the extent of metastasis, overall health, and preferences. The patient and her healthcare team worked together to develop a plan that balanced aggressive treatment with symptom management.The long-term effects of treatment varied significantly depending on the specific therapies used.
Some patients may experience side effects such as fatigue, nausea, hair loss, and difficulty swallowing. Careful monitoring and supportive care are essential to mitigate these side effects. In this case, the patient experienced some side effects from chemotherapy but was able to manage them through supportive care measures. It’s important to note that the long-term effects can also be psychological and emotional, and the patient’s mental well-being was supported throughout the journey.
Last Point
In conclusion, metastatic adenocarcinoma is a complex and challenging disease, but understanding its various facets is crucial for patients, families, and healthcare professionals alike. This overview has provided a comprehensive look at the disease, highlighting the importance of early diagnosis, personalized treatment plans, and the vital role of support systems. By arming ourselves with knowledge, we can navigate the challenges of this disease with greater understanding and empathy.