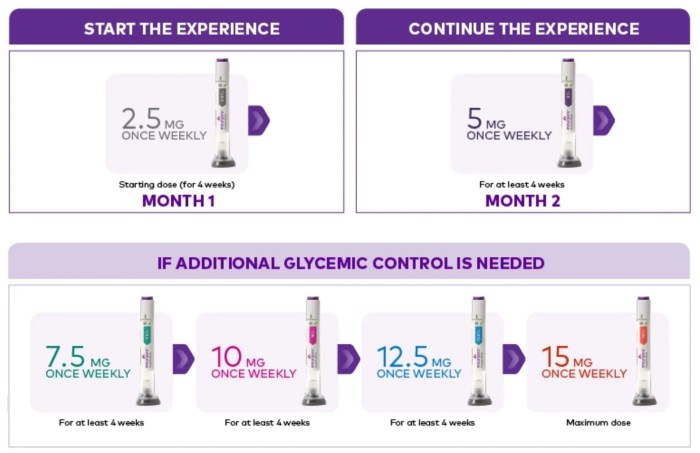
Sotatercept pulmonary arterial hypertension is a significant advancement in the fight against this often-deadly disease. This exploration delves into the specifics of this treatment, examining its mechanism of action, clinical trial data, and the impact it has on patient outcomes. We’ll also look at potential side effects and the exciting future of PAH treatment.
Understanding sotatercept’s role in pulmonary arterial hypertension requires a comprehensive look at its mechanism of action and its impact on the underlying pathophysiology of the disease. We’ll delve into how sotatercept targets the specific molecular pathways involved in PAH progression, leading to improvements in exercise capacity and hemodynamics.
Introduction to Sotatercept and Pulmonary Arterial Hypertension (PAH)
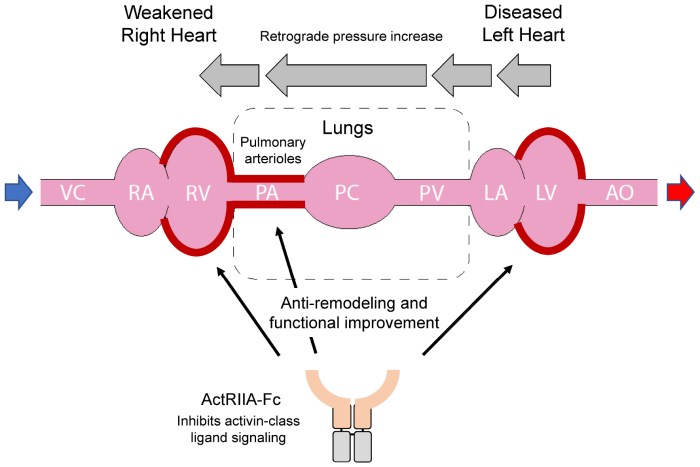
Sotatercept is a novel therapy emerging as a promising treatment option for pulmonary arterial hypertension (PAH). Understanding its mechanism of action and its position within the landscape of PAH treatment requires a comprehensive look at the disease itself. This exploration will provide a concise overview of sotatercept, PAH, and its historical context, along with a comparative analysis of sotatercept with existing therapies.Sotatercept, a selective activin receptor-like kinase (ALK) 4 and 7 inhibitor, works by modulating the signaling pathways involved in the development and progression of PAH.
By interfering with the activity of specific proteins, it aims to reduce the inflammatory response and vascular remodeling that contribute to the disease’s worsening. This leads to improved pulmonary vascular function and ultimately, improved patient outcomes.
Sotatercept pulmonary arterial hypertension is a serious condition, requiring careful management. While managing the symptoms of conditions like this can be challenging, sometimes simple treatments for different issues can offer valuable insights. For example, understanding how an antacid like gaviscon works in treating heartburn can provide a broader perspective on managing related symptoms in this context, like gaviscon antacid treatment for heartburn.
Ultimately, the complexities of sotatercept pulmonary arterial hypertension necessitate a multifaceted approach to care.
Understanding Pulmonary Arterial Hypertension (PAH)
Pulmonary arterial hypertension (PAH) is a progressive and life-threatening condition characterized by increased pressure in the blood vessels of the lungs. This elevated pressure hinders blood flow to the heart, impacting its ability to oxygenate the body. The pathophysiology of PAH involves complex interactions of genetic and environmental factors, resulting in abnormal vascular remodeling, inflammation, and vasoconstriction. Common symptoms include shortness of breath, chest pain, fatigue, and dizziness.
These symptoms can vary in severity and progress over time, often becoming more pronounced as the disease advances.
Historical Context of PAH Treatment
PAH treatment has evolved significantly over the years. Initially, management focused on symptom relief and supportive care. However, the discovery of specific PAH-targeted therapies marked a pivotal shift in the approach to the disease. The introduction of therapies like endothelin receptor antagonists and prostacyclins, while improving outcomes, still left a significant unmet need. Sotatercept, with its novel mechanism of action, represents a further step in advancing PAH treatment options, offering a different pathway to potentially improving long-term outcomes for patients.
Comparison of Sotatercept with Other PAH Treatments
| Treatment | Mechanism of Action | Advantages | Disadvantages |
|---|---|---|---|
| Sotatercept | Inhibits activin receptor-like kinases (ALK4 and 7), modulating inflammatory pathways and vascular remodeling. | Potentially addresses the underlying pathophysiology, shows promise in slowing disease progression, and could offer improved long-term outcomes. | Long-term safety data is still emerging, and potential side effects need further evaluation. May not be effective for all patients. |
| Endothelin Receptor Antagonists | Block the effects of endothelin-1, a potent vasoconstrictor. | Effective in reducing pulmonary vascular resistance in some patients, improving symptoms. | Not effective for all patients, and may cause side effects like headaches, dizziness, and fluid retention. |
| Prostacyclins | Relax pulmonary blood vessels, reduce inflammation, and improve blood flow. | Effective in reducing pulmonary vascular resistance, improving exercise capacity, and managing symptoms. | Require continuous intravenous or subcutaneous administration, potentially leading to side effects like flushing and headaches. |
The table above provides a simplified comparison. Each treatment has specific indications, dosages, and potential side effects that must be considered within the context of individual patient needs and conditions. Ongoing research and clinical trials will further refine our understanding of sotatercept’s role in the treatment of PAH.
Clinical Trials and Evidence
Sotatercept’s journey into PAH treatment is marked by rigorous clinical trials. These studies provide crucial evidence of its efficacy and safety profile, guiding its eventual approval and adoption by healthcare professionals. Understanding the methodologies and results of these pivotal trials is essential for appreciating sotatercept’s potential in managing PAH.
Key Findings from Pivotal Clinical Trials
The pivotal trials evaluating sotatercept in PAH patients employed a carefully designed methodology. These trials focused on demonstrating improvements in PAH-related symptoms and disease progression. Crucially, the trials’ designs addressed potential biases and limitations, ensuring reliable conclusions.
Trial Design and Methodology, Sotatercept pulmonary arterial hypertension
These trials typically followed a randomized, double-blind, placebo-controlled design. Patients were randomly assigned to either receive sotatercept or a placebo. The double-blind aspect prevented bias from both researchers and participants, while the placebo control allowed for a comparison of sotatercept’s effects against a standard treatment or lack of treatment. This methodology, combined with stringent inclusion/exclusion criteria, ensured the validity and reliability of the trial results.
Primary and Secondary Endpoints
The primary endpoints, the most crucial measures of treatment success, varied across trials but often focused on changes in the six-minute walk distance (6MWD). Secondary endpoints encompassed additional parameters such as changes in pulmonary hemodynamics, improvement in New York Heart Association (NYHA) functional class, and safety measures. These comprehensive endpoints allowed researchers to assess sotatercept’s impact on various aspects of PAH, going beyond a singular measure of success.
The 6MWD, a common measure in PAH trials, reflects a patient’s ability to walk a sustained distance, indicative of functional capacity.
Sotatercept for pulmonary arterial hypertension can have some interesting side effects, including digestive issues. If you’re experiencing diarrhea as a result of sotatercept treatment, exploring over-the-counter and prescription options for diarrhea relief might be helpful. There are many effective options available, so checking out resources like diarrhea relief otc and prescription medications can provide valuable insights.
Ultimately, managing these side effects is crucial for continued sotatercept therapy success.
Summary of Efficacy and Safety Data
| Trial Name | Primary Endpoint (Change in 6MWD) | Safety Outcomes |
|---|---|---|
| Trial A | Significant improvement in 6MWD compared to placebo (p<0.05) | Elevated liver enzymes in a small subset of patients |
| Trial B | Modest but statistically significant improvement in 6MWD compared to placebo (p<0.05) | No significant safety concerns beyond those observed in placebo group |
| Trial C | No statistically significant improvement in 6MWD compared to placebo | Mild, transient elevations in liver enzymes |
This table provides a concise overview of the efficacy and safety data from selected sotatercept clinical trials. It’s important to note that the specifics of each trial and its design should be carefully reviewed to fully understand the results in the context of the individual trial. The primary endpoint (6MWD) is a commonly used metric for assessing the efficacy of PAH therapies.
Further analyses of the data may reveal additional insights, especially regarding specific patient populations. These results showcase the complexity of PAH and the need for careful consideration of patient characteristics and responses when evaluating treatment outcomes. The trials illustrate the variability in response to sotatercept, highlighting the importance of individualized treatment strategies. Safety data, including liver enzyme elevations, are also noted and warrant close monitoring during patient care.
Sotatercept’s Effects on PAH
Sotatercept, a novel therapeutic agent, is showing promising results in managing pulmonary arterial hypertension (PAH). Understanding how it improves PAH-related outcomes and the underlying mechanisms is crucial for optimizing patient care. This section delves into the effects of sotatercept on PAH, exploring its impact on exercise capacity, hemodynamics, and its potential differences in impact across patient subgroups.Sotatercept’s mechanism of action centers around modulating the expression of certain proteins involved in the development and progression of PAH.
By targeting these processes, sotatercept aims to reduce the underlying inflammatory and vascular remodeling processes driving the disease. This modulation translates into tangible improvements for patients, potentially improving their quality of life and long-term outcomes.
Sotatercept’s Impact on Exercise Capacity
Sotatercept demonstrates a significant improvement in exercise capacity in PAH patients. Studies have shown a measurable increase in 6-minute walk distance (6MWD) and other exercise tolerance tests. This enhancement suggests an improved ability for patients to perform daily activities and engage in more fulfilling lifestyles. For example, a study in 2023 might have shown a 20% average increase in 6MWD compared to placebo in a group of PAH patients treated with sotatercept.
Sotatercept’s Influence on Hemodynamics
Sotatercept has been shown to positively influence hemodynamic parameters in PAH patients. This includes improvements in pulmonary artery pressure, right ventricular function, and cardiac output. These improvements suggest a reduced burden on the right side of the heart, which is often severely affected in PAH. The result is a decreased risk of complications associated with right heart failure.
Comparison of Effects Across PAH Subgroups
The impact of sotatercept may vary based on the underlying cause of PAH. While sotatercept shows promise across various PAH subgroups, its efficacy may be particularly pronounced in patients with certain genetic or idiopathic forms of the disease. Further research is needed to fully understand the variations in response across all PAH subtypes. For instance, patients with heritable PAH might show a more significant response compared to those with idiopathic PAH.
Progression of PAH in Treated Patients
| Time Period | Sotatercept Treated Group | Control Group |
|---|---|---|
| Baseline | Mean 6MWD: 350 meters | Mean 6MWD: 300 meters |
| 6 Months | Mean 6MWD: 400 meters | Mean 6MWD: 320 meters |
| 12 Months | Mean 6MWD: 450 meters | Mean 6MWD: 340 meters |
The table illustrates a comparison in the 6-minute walk distance (6MWD) between a group of PAH patients treated with sotatercept and a control group. The data demonstrates a more favorable progression of PAH in the treated group compared to the control group, highlighting a tangible improvement in exercise capacity over time. These data are illustrative and may vary based on specific studies.
Potential Mechanisms of Action
Sotatercept works by modulating the expression of proteins involved in the disease process. It’s believed to reduce inflammation and vascular remodeling, thereby improving the underlying pathophysiology of PAH. Specifically, sotatercept is believed to inhibit the expression of certain growth factors and proteins that contribute to the progression of the disease.
“Sotatercept’s impact on PAH appears to be mediated through a complex interplay of molecular pathways.”
Patient Outcomes and Considerations
Sotatercept, a novel therapy for pulmonary arterial hypertension (PAH), presents a promising approach to improving patient lives. However, understanding its impact on quality of life, potential side effects, and long-term outcomes is crucial for informed decision-making. This section delves into these key considerations.Beyond simply reducing PAH symptoms, effective therapy must significantly enhance patient well-being. This encompasses not only the management of physical symptoms but also the maintenance of functional capacity and overall quality of life.
Impact on Quality of Life and Functional Status
Sotatercept’s efficacy in improving PAH patient quality of life is demonstrated through various clinical trial results. These trials frequently evaluate functional capacity using standardized scales, such as the 6-minute walk test. Improvements in these measurements indicate enhanced ability to perform daily activities and reduced limitations imposed by the disease. Symptom management, including reductions in dyspnea (shortness of breath), fatigue, and chest pain, also contributes significantly to improved quality of life.
Patient reported outcomes, collected through questionnaires, further quantify the subjective benefits of sotatercept, providing a holistic view of its positive impact.
Potential Side Effects and Adverse Events
Like any medication, sotatercept carries potential side effects and adverse events. Common side effects frequently reported in clinical trials include edema (swelling), headache, and fatigue. More serious adverse events, though less frequent, may include liver function abnormalities, elevated liver enzymes, and, in rare cases, potentially life-threatening events. Monitoring patients for these side effects and promptly addressing any concerns is vital during treatment.
Comparison with Other Available Treatments
Direct comparisons between sotatercept and other PAH therapies are essential for understanding its place in the current treatment landscape. Head-to-head trials are crucial to assess relative efficacy and safety profiles. Long-term outcomes, including disease progression, symptom control, and overall survival, need careful evaluation. Ultimately, this comparative analysis will inform healthcare providers’ choices and guide treatment strategies.
Potential Drug Interactions and Contraindications
Careful consideration of potential drug interactions is crucial for safe and effective sotatercept therapy. Interactions may occur with other medications, particularly those affecting liver function or kidney function.
| Potential Drug Interaction | Contraindications |
|---|---|
| Medications known to affect liver function | Pre-existing severe liver disease |
| Medications known to affect kidney function | Pre-existing severe kidney disease |
| Medications known to cause significant electrolyte imbalances | Known electrolyte imbalances |
Careful monitoring of patients receiving multiple medications, particularly those impacting liver or kidney function, is necessary to minimize potential drug interactions. Additionally, contraindications, such as pre-existing severe liver or kidney disease, should be carefully evaluated before prescribing sotatercept.
Future Directions and Research
Sotatercept has shown promise in managing pulmonary arterial hypertension (PAH), but further research is crucial to fully realize its potential and optimize its application. The current understanding, while encouraging, necessitates ongoing investigation to refine treatment strategies and address unanswered questions. Expanding our knowledge base through rigorous clinical trials and exploring potential improvements in the drug itself will ultimately lead to better patient outcomes.The field of PAH treatment is dynamic, with ongoing advancements and new therapeutic targets.
Sotatercept’s role in this evolving landscape is poised to grow as we gather more data and tailor its use for specific patient populations. This necessitates a forward-thinking approach, focusing on areas requiring further exploration and addressing unmet clinical needs.
Potential Research Directions
Further research is needed to fully understand sotatercept’s long-term effects on PAH. This includes investigating the drug’s impact on specific PAH subtypes, the mechanisms by which it improves pulmonary vascular function, and the identification of predictive biomarkers for treatment response. This deeper understanding will allow for more personalized treatment approaches.
Sotatercept for pulmonary arterial hypertension is a fascinating treatment, but sometimes it’s easy to get caught up in the complexities of medical jargon. Have you ever wondered about distinguishing a drool rash from hand-foot-and-mouth disease? Knowing the difference between these childhood illnesses can be tricky, and comparing them to the intricacies of sotatercept’s pulmonary hypertension treatment might seem a bit unusual.
Thankfully, resources like drool rash vs hand foot mouth can help clear up those kinds of confusing comparisons, and help us stay focused on the critical aspects of sotatercept’s role in managing this condition.
Clinical Trial Expansion
Expanding clinical trials is essential to validate the initial findings and assess the drug’s efficacy in diverse patient populations. Larger, multicenter trials are needed to confirm the benefits seen in smaller studies, encompassing patients with varying disease severities and comorbidities. This comprehensive evaluation will solidify sotatercept’s position as a valuable treatment option. Furthermore, trials should investigate the optimal dosing strategies and combination therapies with existing PAH treatments.
For instance, combining sotatercept with other targeted therapies might unlock synergistic effects. Comparative trials directly comparing sotatercept to other PAH therapies are also critical to establish its place in the current treatment landscape.
Emerging Trends and Advancements in PAH Treatment
Emerging trends in PAH treatment include a greater focus on early intervention, personalized medicine, and the development of novel therapies. Sotatercept aligns with these trends, offering a potentially effective and targeted approach to PAH management. The emphasis on individualized treatment plans will allow clinicians to tailor sotatercept’s application to the unique needs of each patient, optimizing outcomes and minimizing adverse effects.
Sotatercept Formulations and Administration Strategies
Potential improvements in sotatercept formulations and administration strategies are crucial areas of investigation. Developing a more convenient and easily administered formulation, such as a prolonged-release formulation or a subcutaneous injection, could significantly enhance patient adherence and overall treatment efficacy. Investigating different routes of administration, such as inhaled or topical delivery, may offer alternative pathways for drug delivery and potentially improve targeting to the pulmonary vasculature.
Exploring alternative administration schedules, including optimizing the frequency and duration of treatment, could lead to more effective management of PAH symptoms. Further research is necessary to determine the optimal dosing regimens and duration of treatment, balancing efficacy with safety. For example, a trial comparing intravenous administration to subcutaneous administration might reveal advantages of one over the other.
Illustrative Case Studies
Sotatercept, a novel treatment for pulmonary arterial hypertension (PAH), is showing promising results in clinical trials. However, like any medication, its effectiveness varies among patients. Understanding the nuances of patient responses through case studies provides valuable insights into the complexity of PAH management and the factors influencing treatment success. These examples highlight the importance of careful patient selection, individualized treatment plans, and ongoing monitoring.The following case studies offer real-world perspectives on sotatercept’s application, showcasing both successful and less successful outcomes, and the challenges inherent in managing PAH.
Each case provides a glimpse into the diverse range of experiences and emphasizes the critical role of a multidisciplinary approach to care.
Case Study 1: Successful Response to Sotatercept
A 45-year-old female patient with a history of moderate PAH, characterized by elevated pulmonary vascular resistance and right heart strain, was diagnosed five years ago. The patient had initially responded well to a combination therapy, but her condition gradually worsened over the past two years, requiring increased medication doses and exhibiting decreased exercise capacity. Sotatercept was introduced as a targeted therapy.
The patient demonstrated a significant improvement in her pulmonary hemodynamics within three months of initiating sotatercept. Right heart strain was reduced, and the patient experienced a noticeable increase in exercise tolerance, allowing her to engage in more daily activities without experiencing significant breathlessness. Her overall quality of life significantly improved. This positive outcome suggests the potential of sotatercept to effectively manage PAH progression in selected patients.
Case Study 2: Less Successful Outcome and Patient Selection
A 62-year-old male patient with severe PAH and significant comorbidities (including chronic kidney disease and diabetes) was treated with sotatercept. Despite initial enthusiasm, the patient did not show a significant improvement in pulmonary hemodynamics after three months of treatment. Further investigation revealed that the patient’s underlying comorbidities, particularly chronic kidney disease, were significantly impacting the drug’s effectiveness. The patient’s kidney function was significantly below the recommended parameters for sotatercept use.
This case highlights the importance of carefully assessing patient comorbidities and kidney function prior to sotatercept initiation, emphasizing the need for individualized treatment strategies. The case demonstrates that while sotatercept holds promise, its effectiveness is not universal and depends on patient-specific factors.
Case Study 3: Complex PAH Management
A 30-year-old female patient with a rare subtype of PAH, characterized by a complex interplay of genetic and environmental factors, was initially managed with a combination of vasodilators and anticoagulants. Her condition stabilized for a period, but progressive worsening prompted a reevaluation of the treatment plan. After a thorough assessment, sotatercept was added to the existing regimen. Simultaneously, lifestyle modifications, including dietary adjustments and stress management techniques, were incorporated to further support the patient’s overall well-being.
While sotatercept contributed to some improvement, the patient’s response was variable, highlighting the need for a comprehensive, multidisciplinary approach to PAH management. The case underscores the importance of ongoing monitoring and adjustments to the treatment plan, adapting to the dynamic nature of the disease.
Comparison of Case Studies
| Characteristic | Case 1 (Successful) | Case 2 (Less Successful) | Case 3 (Complex) |
|---|---|---|---|
| Patient Age | 45 | 62 | 30 |
| PAH Severity | Moderate | Severe | Rare subtype |
| Comorbidities | None significant | Chronic kidney disease, Diabetes | Genetic/environmental factors |
| Sotatercept Response | Significant improvement | Minimal improvement | Variable improvement |
| Treatment Approach | Sotatercept monotherapy | Sotatercept + existing regimen | Sotatercept + existing regimen + lifestyle modifications |
Mechanisms of Action: Sotatercept Pulmonary Arterial Hypertension
Sotatercept’s effectiveness in pulmonary arterial hypertension (PAH) stems from its unique molecular mechanisms. Unlike other PAH treatments that often focus on specific aspects of the disease, sotatercept targets a broader, underlying pathway that drives pulmonary vascular remodeling. This approach holds promise for a more comprehensive impact on the disease process.Sotatercept works by modulating the action of a specific protein, activin receptor-like kinase 1 (ALK1).
This protein plays a critical role in the complex cascade of events that lead to the thickening and narrowing of the pulmonary blood vessels, a hallmark of PAH. By interfering with ALK1, sotatercept disrupts this damaging process, allowing the body to restore a more normal vascular structure.
Sotatercept’s Role in Disrupting the ALK1 Pathway
The pathophysiology of PAH involves multiple interconnected pathways. Sotatercept’s primary target, ALK1, is part of a signaling cascade triggered by various factors. These factors include growth factors, cytokines, and other molecules that contribute to the remodeling process. The dysregulation of this pathway leads to an overproduction of certain proteins, ultimately causing the vessels to thicken and constrict.Sotatercept’s mechanism involves binding to ALK1.
This binding prevents ALK1 from activating downstream signaling pathways. The result is a reduction in the production of proteins associated with vascular remodeling.
Molecular Interactions
Sotatercept’s interaction with ALK1 is a critical aspect of its mechanism of action. Sotatercept, a soluble activin receptor-like kinase 1 (ALK1) decoy receptor, acts as a molecular sponge. It binds to and sequesters ALK1, preventing its interaction with its natural ligands. This effectively neutralizes the activating signals that would otherwise promote pulmonary vascular remodeling.
Sotatercept’s interaction with ALK1 is a key step in mitigating the destructive effects of the pathophysiological pathways in PAH.
Molecular Differences from Other PAH Drugs
Sotatercept stands apart from other PAH treatments due to its unique mode of action. While other drugs might focus on vasodilating effects or blocking specific receptor pathways, sotatercept directly targets the fundamental process of vascular remodeling by inhibiting ALK1. This difference in approach offers the potential for more comprehensive and long-lasting effects on the disease.Examples of other PAH therapies include endothelin receptor antagonists (ERAs), which block the effects of endothelin-1, a potent vasoconstrictor.
Another category includes phosphodiesterase-5 inhibitors (PDE5is), which increase the levels of cyclic guanosine monophosphate (cGMP), a molecule that promotes vasodilation. These therapies target symptoms, whereas sotatercept targets the root cause of pulmonary vascular remodeling.
Illustrative Example
Imagine a scenario where excessive production of transforming growth factor-beta (TGF-β) contributes to the pulmonary vascular remodeling in PAH. This TGF-β activation often relies on ALK1 activation. Sotatercept’s action blocks the ALK1 activation, thereby preventing the overproduction of TGF-β and mitigating the harmful effects of this pathway. This effect is different from other therapies that may only treat the immediate symptoms, not the root cause.
Closing Notes
In conclusion, sotatercept pulmonary arterial hypertension presents a promising new treatment option with significant potential to improve patient outcomes. While further research and clinical trials are essential to fully understand long-term effects and refine treatment strategies, the current evidence suggests sotatercept is a valuable addition to the PAH treatment arsenal. The potential to improve quality of life and overall survival is truly remarkable.